Abstract
Alpha-2 adrenergic receptors (A2AR) regulate multiple brain functions and are enriched in developing brain. Studies demonstrate norepinephrine (NE) plays a role in regulating brain maturation, suggesting it is important in A2AR development. To investigate this we employed models of NE absence and excess during brain development. For decreases in NE we used N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP4), a specific noradrenergic neurotoxin. Increased noradrenergic terminal density was produced by methylazoxymethanol acetate (MAM) treatment. A2AR density was assayed with [3H]RX821002 autoradiography. DSP4 lesions on postnatal day (PND) 3 produce A2AR decreases in many regions by PND 5. A2AR recover to control levels by PND 15 and 25 and there is no further change in total receptor density. We also assayed A2AR in brains lesioned with DSP4 on PND 13, 23, 33 and 43 and harvested 22 days post-lesion. A2AR levels remain similar to control at each of these time points. We examined A2AR functionality and high affinity state with epinephrine-stimulated [35S]GTPγS and [125 I]p-iodoclonidine autoradiography, respectively. On PND 25, control animals and animals lesioned with DSP4 on PND 3 have similar levels of [35S]GTPγS incorporation and no change in high affinity state. This is in contrast to increases in A2AR high affinity state produced by DSP4 lesions of mature brain. We next investigated A2AR response to increases in norepinephrine levels produced by MAM. In contrast to DSP4 lesions, increasing NE results in a large increase in A2AR. Animals treated with MAM on gestational day 14 had cortical [3H]RX821002 binding 100-200% greater than controls on PND 25, 35, 45, 55 and 65. These data indicate that NE regulation of A2AR differs in developing and mature brain and support the idea that NE regulates A2AR development and this has long term effects on A2AR function.
Keywords: Alpha-2 adrenergic receptors, Development, DSP-4, Methylazoxymethanol, Norepinephrine transporter, High affinity receptor, G protein linkage
1
Norepinephrine (NE) is a neurotransmitter that signals through alpha-1, alpha-2 and beta adrenergic receptors. Studies support a role for NE in brain function as well as brain development (Lauder, 1993; Lipton and Kater, 1989). Experiments have further indicated noradrenergic receptors are important in developing as well as mature brain. For instance, noradrenergic signaling through alpha-1 adrenergic receptors has an important role in regulating prefrontal cortical function of adult animals (Ramos and Arnsten, 2007). Beta- adrenergic receptor stimulation plays an important role in memory processes within the amygdala and hippocampus (Bush et al., 2010; Murchison et al., 2011). In the developing rat brain, alpha-1 adrenergic receptors are observed as early as embryonic day 14 suggesting important developmental roles (McCune and Hill, 1995). Beta receptor stimulation in neonatal rats is important for learning-induced plasticity in the olfactory system (Sullivan et al., 1989).
Alpha-2 adrenergic receptors (A2AR) are also implicated in distinct processes within mature as well as developing brain. Alpha-2 adrenergic receptors are widely distributed throughout the central nervous system (CNS) (Nicholas et al., 1993; Unnerstall et al., 1984; Wamsley et al., 1992) and subserve many physiological functions. These G protein-coupled receptors (GPCR) activate Gi/o proteins to inhibit adenylyl cyclase (Duman and Enna, 1986; Woodcock and Johnston, 1982), activate inwardly rectifying K+ channels and modulate voltage-gated calcium channels (Abdulla and Smith, 1997; Huangfu and Guyenet, 1997; Jeong and Ikeda, 2000). A2AR are known to be localized both presynaptically and postsynaptically. Activation of the presynaptic receptors inhibits release of neurotransmitters, including NE, glutamic acid, aspartic acid and GABA (Bucheler et al., 2002; Hein et al., 1999; Kamisaki et al., 1992). Downstream, A2AR activate mitogen-activated protein kinase and negatively regulate immediate early gene (IEG) expression (Shen and Gundlach, 2000; Williams et al., 1998).
The widespread CNS distribution of A2AR and the important roles these receptors play in regulating CNS functions are the bases for their being frequent pharmacological targets for the treatment of pathophysiological states. The A2AR agonist, clonidine, is widely employed as an anti-hypertensive agent due to its actions on cardiovascular regulatory centers in the brainstem (Yamazato et al., 2001). Dexmedetomidine reduces infarct size in models of brain ischemia (Jolkkonen et al., 1999; Matsumoto et al., 1993). A2AR agonists also have been shown to enhance cognition. Guanfacine improves working memory and function of the prefrontal cortex (Arnsten et al., 1996; Franowicz and Arnsten, 1999; Franowicz et al., 2002). Antagonists at A2AR are employed as antidepressants (Davis et al., 2001).
There are three subtypes of A2AR, designated A/D, B and C (Bylund, 1992; Kable et al., 2000). A2AR mRNA is enriched in many developing brain structures, suggesting it plays an important role in brain development. For example, by E19 A2AR-A are expressed at high levels in the cortex, septum and olfactory system at levels equivalent to those seen in adult brain. In areas such as the basal ganglia, amygdala, thalamus, spinal cord and brainstem A2AR-A mRNA is expressed at high levels in early development and then decreases with subsequent maturation of the nervous system (Winzer-Serhan et al., 1997a). A2AR are expressed at very high levels in white matter of developing rat CNS, then disappear in adulthood, indicating a role in regulating development (Sanders et al., 2005b). In cortex of the embryonic rhesus monkey A2AR display a high receptor density which surpasses that for most other receptors (Lidow and Rakic, 1994).
We sought to determine the role of noradrenergic innervation in the development of A2AR. Several studies have demonstrated important roles for NE in developmental processes. These include the regulation of synaptic density, neuronal morphology and IEG expression (Felten et al., 1982; Parnavelas and Blue, 1982; Sanders et al., 2008). Based on this, we hypothesized that norepinephrine plays an important role in regulating the maturation of A2AR. To address this question we employed models of norepinephrine absence and excess during development. To study the effect of decreases in norepinephrine during development we used N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP4) lesions of neonatal rats. DSP4 decreases brain norepinephrine by selectively destroying the terminals of the noradrenergic neurons originating in the locus coeruleus, which provide the majority of brain NE (Jonsson et al., 1982). Increasing NE during brain development was accomplished by the methylazoxymethanol acetate (MAM) lesion model (Johnston et al., 1979). In this study we provide evidence that NE plays an important role in directing A2AR development and that noradrenergic regulation of A2AR differs in the developing and mature brain.
2. EXPERIMENTAL PROCEDURES
2.1. Materials
[3H]RX821002 (58 Ci/mmol: 2-[2-(2-methoxy-1,4benzodioxanyl)]imidazoline hydrochloride) was obtained from Amersham (Arlington Heights, IL). [35S]GTPγS (1000-1500 Ci/mmol), [125I]p-iodoclonidine (2200 Ci/mmol) and [3H]nisoxetine (80 Ci/mmol) were purchased from NEN Life Science Products (Boston, MA). Epinephrine bitartrate, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP4), RX821002, rauwolscine, glycylglycine HCl, dithiothreitol (DTT) and desipramine were purchased from Sigma (St. Louis, MO). Guanosine 5′-diphosphate sodium (GDP) was purchased from United States Biochemical (Cleveland, OH). Methazoxymethanol (MAM) was obtained from NCI (Kansas City, MO). All other chemicals were research grade.
2.2. Animals
Sprague-Dawley rats (Sasco, Kingston, NY) were bred in our colony. For DSP4 studies, animals were treated with 50 mg/kg of DSP4 in sterile saline, i.p. For MAM studies pregnant Sprague-Dawley rats (Sasco, Kingston, NY) were injected with 25mg/kg MAM in sterile saline, i.p., on day14 of gestation. Control animals received saline. Brains were collected and rapidly frozen on dry ice and stored at −80 °C. For studies correlating [3H]nisoxetine binding with NE levels, animals were injected with DSP4 or saline on PND 8 and harvested on PND 32. Procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80–23, revised 1996) and were approved by the UNMC Animal Care Committee. Studies were designed to minimize the number of animals used and their pain and suffering.
2.3 AUTORADIOGRAPHIC PROCEDURES
2.3.1. [3H]RX821002 Autoradiography
Brains from control and DSP4 treated rats were frozen on dry ice and stored at −80°C until use. Brains were sectioned at 16 μm and thaw mounted onto gelatin-subbed slides. Slides were then stored at −80 °C with desiccant. Prior to incubation sections were brought to room temperature (RT) for 45 min. Sections were then incubated in 2.0 nM [3H]RX821002, 50 mM sodium phosphate, pH 7.4 for 1hr at RT. Nonspecific binding was determined by addition of 10 μM rauwolscine. Incubation was followed by two 5 min washes in ice-cold buffer followed by a quick dip in ice cold water to remove salts. Sections were then dried under a stream of cool air and apposed to tritium sensitive film (Hyperfilm-3H, Amersham) for four weeks. Film was developed in Kodak D19 (Rochester, N.Y.) for 5 min, Kodak indicator stop bath for 30s and Kodak Rapid Fixer for 4 min.
The MCID image analysis system (MCID-M7; Interfocus Imaging, Ltd., Linton, England) and commercial tritium standards (American Radiolabeled Chemicals, St. Louis, MO) that were individually calibrated to [3H]-tissue standards were used to quantify receptor densities from films. Neuroanatomy was confirmed by comparison with a brain atlas (Paxinos and Watson, 1998).
2.3.2. [125I]Para-Iodoclonidine Autoradiography
Sections, obtained as described above for [3H]RX821002 autoradiography, were allowed to come to room temperature and were then incubated for 3 hr at RT in 50 mM Tris–HCl, 120 mM sucrose, pH 7.4 with 50 pM [125I]p-iodoclonidine as ligand. Nonspecific binding was determined by addition of 10 μM rauwolscine. Sections were incubated in a horizontal mailer on a shaking water bath platform. Every 15 min mailers were gently inverted three times to ensure homogenous distribution of the radioligand. After incubation sections were washed two times for 5 min in ice-cold 50 mM Tris–HCl, pH 7.4, briefly dipped in ice-cold distilled water and rapidly dried under a stream of cool air. Sections were apposed to Biomax film (Kodak) for 48 h and developed by standard procedures. Autoradiographic densities were quantified based on commercial tritium standards (American Radiochemicals) that were individually calibrated to [125I]-tissue standards (Miller et al., 1988). Neuroanatomy was confirmed by comparison with a brain atlas (Paxinos and Watson, 1998).
2.3.3. [3H]Nisoxetine Autoradiography
[3H]Nisoxetine autoradiography was carried out as previously described (Sanders et al., 2005a). Briefly, 16μm sections were incubated in 2.0 nM [3H]nisoxetine in 10 mM Na2HPO4, 300 mM NaCl and 5 mM KCl, pH 7.4, for 4 h at 4 ºC. Inclusion of 10 μM desipramine defined nonspecific binding. Sections were washed in three separate 5 min washes of ice-cold incubation buffer and dipped in ice-cold water. After being dried under a stream of cool air, slides were apposed to tritium-sensitive film (HyperFilm-3H; Amersham Corp) for 8 weeks. Films were developed in Kodak D19 for 5 min, stop bath for 30s and Kodak Rapid Fixer for 7 min. Images were analyzed with the MCID-M7 image analysis system and autoradiographic densities quantified based on commercial tritium standards (American Radiochemicals, St. Louis, MO) that were individually calibrated to [3H]-tissue standards. Neuroanatomy was confirmed by comparison with a brain atlas (Paxinos and Watson, 1998).
2.3.4. A2AR Agonist-Stimulated [35S]GTP S Autoradiography
Frozen slide-mounted 16μm tissue sections, obtained as described above for [3H]RX821002 autoradiography, were allowed to come to RT for 30 min. They were then hydrated in assay buffer (50 mM glycylglycine, 3 mM MgCl2, 1 mM EGTA, 100 mM NaCl, pH 7.5) at RT for 10 min. This was followed by incubation in assay buffer containing 2 mM GDP for 30 min. Receptor stimulation was carried out for 2 hr in assay buffer containing 2 mM GDP, 0.1 nM [35S]GTPγS, 0.2 mM DTT and 100 μM epinephrine. Addition of 10 μM RX821002, a specific A2AR antagonist, defined the A2AR specificity of receptor stimulation. Sections were washed 2×5 min in ice cold 50mM glycylglycine, 0.2 mM DTT, pH 7.5. Slides were then placed on film (βmax Hyperfilm, Amersham) for 24 h. Autoradiographic densities were quantified based on commercial tritium standards (American Radiochemicals, St. Louis, MO) that were individually calibrated to [35S]-tissue standards (Miller et al., 1988). Neuroanatomy was confirmed by comparison with a brain atlas (Paxinos and Watson, 1998).
2.4. HPLC Analysis Of NE Levels
Endogenous norepinephrine was assayed by high pressure liquid chromatography (HPLC). Freshly isolated cerebral cortex and hippocampus were homogenized in a solution consisting of 2.4% HClO4, 0.1% sodium metabisulfite and 0.05 % Na2EDTA. The homogenized sample was centrifuged at 13,000 rpm for 20–30 min at 4 ºC. The supernatant was stored at −70 ºC until analysis in the laboratory of Dr. Kaushik Patel, Physiology Dept., University of Nebraska Medical Center (Patel et al., 1981).
2.5. Statistics
Control brain areas were compared to norepinephrine deficient areas with a two-tailed t test. Agonist stimulated [35S]GTPγS binding was analyzed by one way ANOVA followed by Tukey’s post hoc test.
3. RESULTS
3.1. NET And NE Are Both Reduced To Near Background By DSP4 In Developing Brain
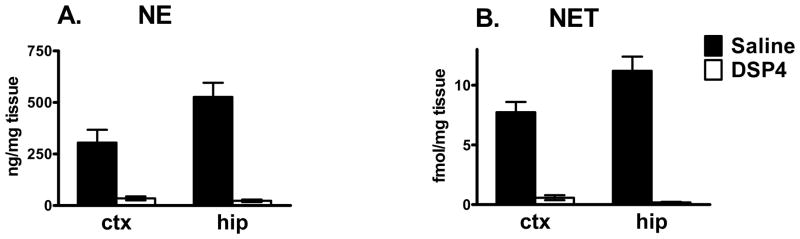
We used the selective noradrenergic neurotoxin, DSP4, to lesion the noradrenergic innervation of developing rat brain. DSP4 destroys noradrenergic terminals emanating from the locus coeruleus (Jaim-Etcheverry and Zieher, 1980; Jonsson et al., 1982), which provides virtually all of the noradrenergic innervation to cortex and hippocampus. Other brain areas, such as the septum and amygdala, receive an overlapping innervation from the locus coeruleus and other brainstem noradrenergic nuclei (Lindvall and Björklund, 1983; Moore and Bloom, 1979) and are affected to a lesser extent by DSP4. Autoradiographic analysis with [3H]nisoxetine, a highly specific ligand for noradrenergic transporters (NET) and hence noradrenergic terminals (Wong and Bymaster, 1976; Wong et al., 1982), was used to verify the loss of noradrenergic terminals. We also determined the extent to which decreases in [3H]nisoxetine binding correlated with decreases in NE in developing brain. Treating neonatal animals with DSP4 and harvesting brains on PND 32 produces a near complete elimination of NE in cerebral cortex and hippocampus (Fig. 1A). This decrease is accompanied by a parallel reduction in NET in both regions (Fig. 1B). This is in agreement with similar studies in adult brain (Tejani-Butt, 1992) and demonstrates that the effects of DSP4 in adult brain are also produced in developing brain. Studies in adult animals further support the parallel between loss of norepinephrine due to DSP4 lesion and loss of [3H]nisoxetine binding (Cheetham et al., 1996). It is a strength of the autoradiographic approach that levels of norepinephrine or of [3H]nisoxetine binding can be demonstrated in sections from the same area as A2AR data are derived.
FIGURE 1.
Reduction in NET parallels reduction in norepinephrine levels in DSP4 treated animals. Neonatal treatment with DSP4 reduces NET and norepinephrine levels in cortex and hippocampus at PND 32 to <5% of control levels. NET was quantified by [3H]nisoxetine binding. NE levels were measured by HPLC. ctx = cortex, hip=hippocampus. Data are mean ± SEM, n = 4. All DSP4 values were significantly different from the corresponding saline controls, p<0.001.
3.2. Rat A2AR Distribution And Density Develop Independently Of NE
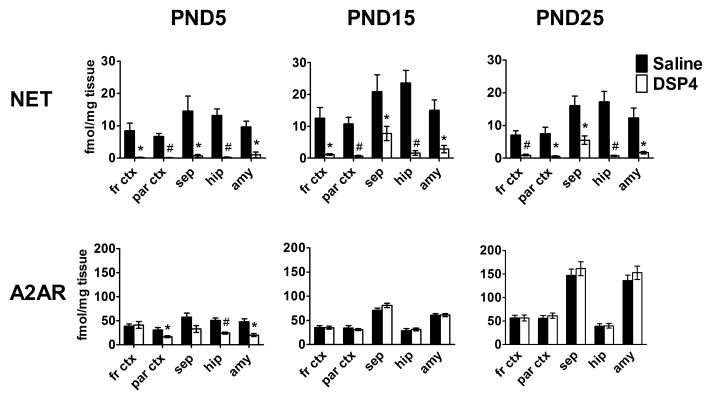
In adult animals denervation or chronic antagonist treatment of many neurotransmitter receptors leads to a compensatory sensitization of the receptor, frequently characterized by expression of more cell surface receptors, or receptor up-regulation (Dooley et al., 1983). We assessed the effects of neonatal noradrenergic depletion on brain A2AR levels during development using quantitative autoradiography. Rats were lesioned with DSP4 on PND 3 and brains were harvested on either PND 5, 15 or 25. Adjacent sections were used to measure NET to confirm lesion efficacy. Only animals with a confirmed depletion of NET greater than 90% within the cortex and hippocampus compared to controls were used for further studies.
Control brain A2AR densities of approximately 40–55 fmol/mg tissue are found across PND 5 brain areas. (Fig 2). By PND 25 cortical A2AR density is approximately 55 fmol/mg tissue while hippocampal fissure A2AR density remains near 40 fmol/mg tissue. Densities in the amygdala and septum are approximately 150 fmol/mg tissue (Fig 2).
FIGURE 2.
Effects of neonatal DSP4 lesion on development of NET and A2AR. DSP4 treatment on PND 3 greatly reduces NET from PND 5 to PND 25 as measured by [3H]nisoxetine binding. A2AR density, as measured by [3H]RX821002 binding, is reduced in some regions two days later (PND 5) but recovers and is not significantly changed compared to controls from PND 15 on. Data for frontal cortex (fr ctx) and septum (sep) are from coronal level 0.7 mm anterior to the bregma while data for parietal cortex (par ctx), amygdala (amy) and hippocampus (hip) are from coronal level 3.3 mm posterior to the bregma, corresponding to plates 15 and 33 in Paxinos and Watson (1998). fr ctx=frontal cortex; sep=lateral septum. Data are mean ± SEM, n = 4–12.
Groups significantly different from saline control, * − p < 0.05; # − p < 0.01.
In general, control brains exhibit the greatest values for NET within the septum and hippocampus and moderately lower densities are observed in the cortex and amygdala. Neonatal DSP4 decreases NET throughout these areas. This effect is most pronounced in the cerebral cortex and hippocampus, followed by the amygdala and septum. The presence of persistent NET in the amygdala and septum reflects the overlapping noradrenergic innervation of these regions by the dorsal medullary and lateral tegmental noradrenergic systems that are spared the effects of DSP4 (Fig. 2). Surprisingly, although NET and NE are dramatically reduced in many brain regions of animals treated with DSP4 neonatally, A2AR densities are not altered and are similar to control values across all brain areas surveyed. At PND 5 there is an approximately 50% decrease in A2AR across all brain regions assayed except the frontal cortex.. These return to control levels in PND15 and PND 25 animals (Fig. 2).
3.3. Effect Of Neonatal Versus Adult Administration Of DSP4 On A2AR Agonist High Affinity State
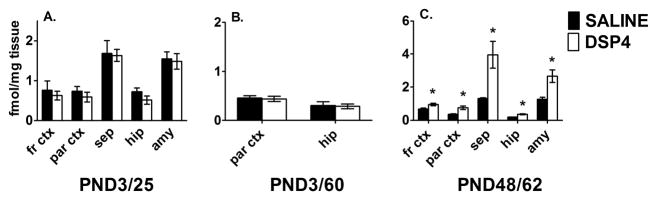
Receptors also may become more sensitive through an increase in agonist affinity or in the percentage of receptors in the agonist high-affinity state. We determined whether the number of A2AR in the agonist high affinity state increases as a consequence of loss of norepinephrine during development despite lack of change in total receptor number. We used [125I]p-iodoclonidine, an A2AR agonist which selectively binds to the agonist high affinity state of A2AR when used at very low concentrations.
In PND 25 control rat brain [125I]p-iodoclonidine binding is highest in the amygdala and septum. Lower levels are measured throughout the cortex and in the hippocampal fissure (Fig. 3A). In control brains the density of [125I]p-iodoclonidine binding on PND 25 parallels NET and total A2AR density (Figs. 2, 3A). High levels of [125I]p-iodoclonidine binding and [3H]RX821002 binding are found in the septum with a correspondingly robust level of [3H]nisoxetine binding. Lower levels of [3H]nisoxetine binding in the cerebral cortex are accompanied by lower [125I]p-iodoclonidine and [3H]RX821002 binding (Fig 3A). This comparison of transporter and A2AR suggests that the density of noradrenergic innervation regulates tissue expression of A2AR and the level of A2AR in the agonist high affinity state. However, similar to [3H]RX821002 binding, [125I]p-iodoclonidine binding does not change in the face of a long-standing norepinephrine depletion in the developing brain. Values similar to control are found across brain regions for DSP4-treated neonates examined on PND 25 (Fig. 3A) and PND 60 (Fig. 3B).
FIGURE 3.
Effects of DSP4 lesion on A2AR agonist high affinity state in developing and mature brain. DSP4 treatment on PND 3 produces no significant change in the level of high affinity state A2AR on PND 25 (A) or PND 60 (B). In contrast, DSP4 treatment on PND 48 produces significant increases in levels of high affinity state A2AR on PND 62 (C). Data are expressed in fmol/mg tissue and are mean ± S.E.M., n=3–8. Data for frontal cortex (fr ctx) and septum (sep) are from the coronal level 0.7 mm anterior to the bregma (3A, 3C) while data for parietal cortex (par ctx), amygdala (amy) and hippocampus (hip) are from coronal level 3.3 mm posterior to the bregma (3A, 3B, 3C: Paxinos and Watson, 1998). For 3B amygdaloid tissue was lost during processing.
* - significantly different from corresponding saline control, p < 0.02.
We also examined high affinity A2AR response in more mature animals by lesioning with DSP4 on PND 48 and examining brains on PND 62. In contrast to brains lesioned on PND 3 and harvested on PND 25 or 60, brains lesioned on PND 48 and harvested on PND 62 exhibit a pronounced up-regulation of [125I]p-iodoclonidine binding in all brain regions examined (Fig. 3C).
3.4. A2AR Agonist-Stimulated [35S]GTPγS Binding Develops Independently Of Norepinephrine
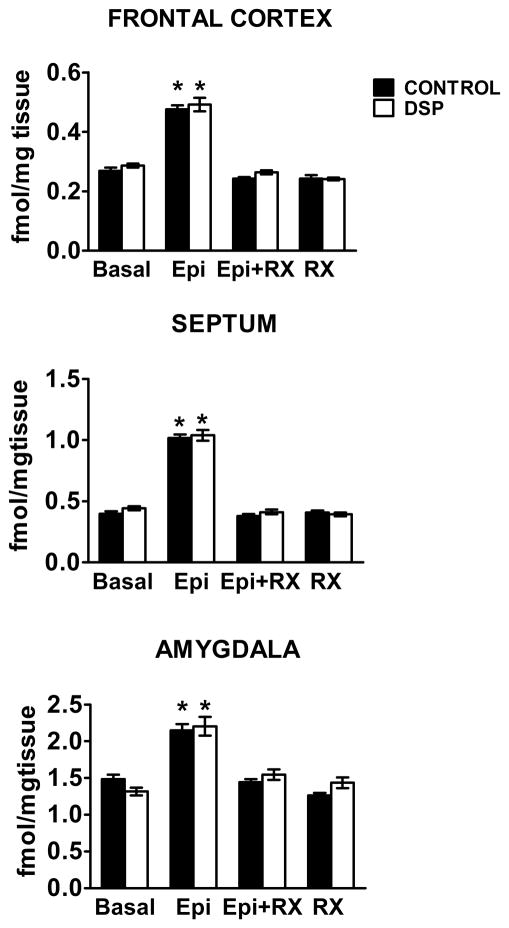
We were surprised to see no change in A2AR density or in percentage of receptors in the agonist high affinity state following DSP4 lesion in neonatal animals, a contrast to adults. We next examined whether there is a change in receptor coupling to G proteins, the first step in the signal transduction pathway for A2AR, using agonist-stimulated [35S]GTPγS binding (Happe et al., 1999). This addressed the question of whether there is increased function of A2AR in animals that had lost noradrenergic innervation during postnatal development even though there is no change in receptor number.
Control animals and animals lesioned with DSP4 neonatally are similar in basal [35S]GTPγS binding across brain regions and demonstrate similar levels of [35S]GTPγS incorporation in response to epinephrine stimulation (Fig. 4). For both control and DSP4-treated animals, the epinephrine-induced increase in [35S]GTPγS binding above basal levels is ~75% for frontal cortex, ~30% for amygdala and ~140% for septum (Fig. 4 ). Addition of 10 μM RX821002 is equally effective in blocking epinephrine stimulation across brain regions in control and DSP4 treated animals. Addition of 10 μM RX821002 alone was assayed for its effect on [35S]GTPγS binding. Although RX821002 is an inverse agonist at A2AR (Murrin et al., 2000; Wade et al., 2001) and theoretically could reduce the incorporation of [35S]GTPγS by A2AR, it had no measureable effect in our assays. The similarity in epinephrine-induced [35S]GTPγS incorporation between treatment groups shows that a second messenger sensitization of A2AR, at least at the level of G protein coupling, does not occur in the developmental absence of norepinephrine.
FIGURE 4.
Linkage of A2AR to G proteins is not affected by neonatal DSP4 lesion. Rats were treated with DSP4 at PND 3 and linkage of A2AR to Gi/o was examined at PND 25 using the [35S]GTPγS assay as described in Methods. Treatment with epinephrine (Epi) increased [35S]GTPγS binding in all brain regions, similar to previously published studies in adults. Neonatal treatment with DSP4 did not alter [35S]GTPγS binding in either control or DSP4 treated animals. There were no significant differences between control (saline) and DSP4 treated animals within any treatment. n= 10–16.
* - significantly different from the corresponding control, RX and Epi+RX, p < 0.001.
3.5. Effect Of Developmental Increase In Norepinephrine On Expression Of A2AR
We found that neonatal lesion of noradrenergic neurons with DSP4 did not alter A2AR levels or linkage to G proteins, indicating that the expression level and functional linkage of A2AR is not altered by the absence of NE during development. We next examined the effect of a developmental increase in NE levels on A2AR expression. We used methylazoxymethanol acetate (MAM), an alkylating agent that destroys dividing cells to increase brain levels of NE. Administration of MAM on E14 to pregnant dams results in a noradrenergic hyper-innervation of the cerebral cortex (Johnston et al., 1981a). The injection of MAM at E14 destroys the dividing cells in the superficial layers of the cerebral cortex, leaving the cells of the locus coeruleus and the deep cortical layers unaffected because they have differentiated before E14 (Johnston et al., 1981a; Luhmann et al., 2003). Because the noradrenergic innervation is unaffected but its projection field is reduced in volume, the effect is a noradrenergic hyperinnervation of the cerebral cortex, resulting in a ~220% increase in the concentration of norepinephrine (Johnston et al., 1981a). Excitatory and inhibitory neurotransmitters and enzymes that are intrinsic to the projection field, such as glutamate decarboxylase and γ-amino-butyric acid, are minimally affected (Johnston et al., 1981a; Johnston et al., 1979). Because MAM eliminates superficial layers 2–4 of the cerebral cortex and preserves layers 5 and 6, this study expresses the NET and A2AR densities of MAM cortex as a percentage of the levels measured in the corresponding cortical layers 5 and 6 of control brains.
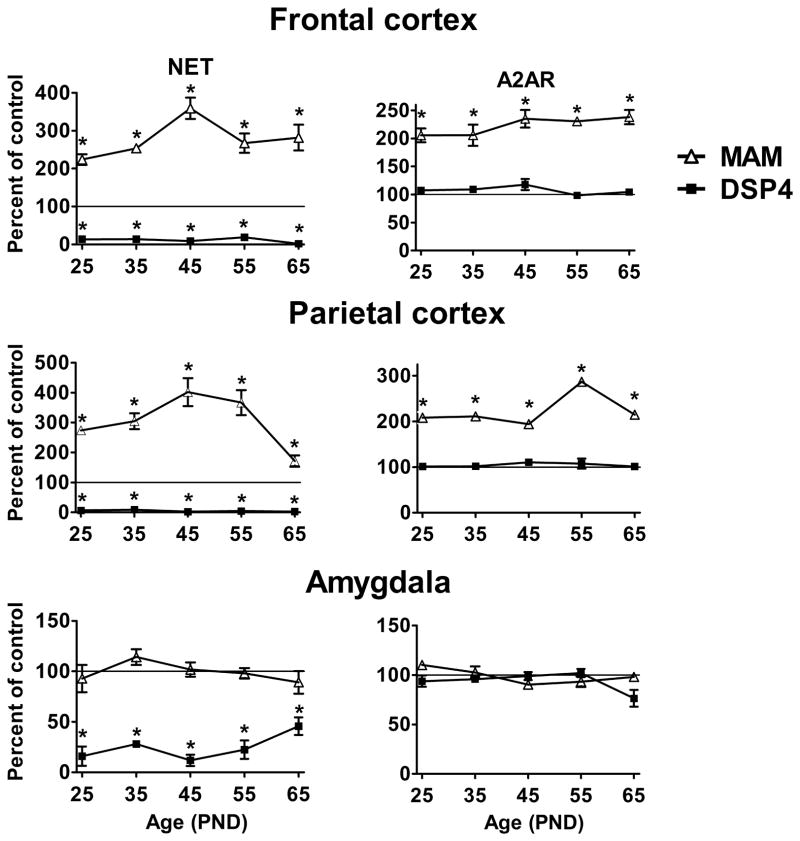
Brains from MAM-treated animals exhibit a dramatic increase in NET density, 100–300% greater than control throughout postnatal development. In frontal cortex at PND 25 and 35 NET levels are 100% greater than control levels (Fig. 5). By PND 45 the levels peak at 250% greater than control and then decline to near 150% greater by PND 55 and 65. A similar pattern of expression is found in parietal cortex where a NET density 180% greater than control was seen at PND 25 and a peak at 300% greater than control at PND 45, In the anteromedial amygdala, a region in which DSP4 reduces NET by 55–80%, MAM did not alter NET expression. This is consistent with the fact that this brain region undergoes the majority of its neurogenesis by E13, thus escaping the effects of MAM (Bayer, 1980).
FIGURE 5.
Comparison of the effects of developmental noradrenergic depletion and excess on NET and A2AR. NET was assayed with [3H]nisoxetine and A2AR with [3H]RX821002. MAM was administered to pregnant dams on E14 and offspring brains harvested at PND 25, 35, 45, 55 and 65. DSP4 was administered 22 days prior to each of the experimental ages. NE hypoinnervation during development due to DSP4 lesions reduced NET levels to near zero but had no effect on A2AR, in contrast to similar lesions in adults. NE hyperinnervation during development due to MAM lesions significantly increased both NET and A2AR levels in cortex. Data are mean ± SEM, n = 4–6.
* - significantly different from the corresponding control, p < 0.01.
In contrast to DSP4, we found that increasing the density of noradrenergic innervation during development results in a large increase in A2AR. [3H]RX821002 binding in the frontal cortex was 100% greater than control values at PND 25 (Fig 5). From PND 45 onward, expression of A2AR in MAM-treated animals is consistently 120% above control values. In the parietal cortex A2AR are generally 100% above control levels with a peak at PND 55 that is 200% greater than control. The amygdala of MAM-treated animals does not have an increase in A2AR. This indicates that the increases in A2AR in MAM-treated animals are localized to areas of increased noradrenergic innervation and therefore it is unlikely that they are a non-specific effect of the toxin.
4. DISCUSSION
Alpha-2 adrenergic receptor signaling is an important component in a variety of behavioral and physiological actions, including learning and memory. The A2AR-B receptor appears to be important in emotional memory (de Quervain et al., 2007). Local administration of the A2AR antagonist yohimbine, which increases NE release from nerve terminals, increases c-fos within the cerebral cortex (Stone et al., 1993), an indication of increased neuronal activity. The antagonist, RX821002, increases zif268, an IEG involved in learning and memory, in cortex, hippocampus and amygdala (Shen and Gundlach, 2000; Shen et al., 1995). Administration of the A2AR agonist, guanfacine, improves working memory in mice, monkeys, and humans (Franowicz and Arnsten, 1999; Franowicz et al., 2002). Transgenic mice which lack one or a combination of the three A2AR subtypes display many behavioral perturbations, including increased immobility in the forced swim test, lack of response to antidepressants, an exaggerated startle reactivity and reduced pre-pulse inhibition of startle responses (Kable et al., 2000; see MacDonald et al., 1997).
A role for A2AR during development is suggested by early mRNA expression and protein enrichment in developing brain (Happe et al., 2004; Lidow and Rakic, 1994; Sanders et al., 2005b; Winzer-Serhan and Leslie, 1997; Winzer-Serhan et al., 1997a; Winzer-Serhan et al., 1997b). Behavioral studies with transgenic A2AR animals also support a role for these receptors in the development and final expression of important CNS functions. Both A2AR-2A and A2AR-2C regulate memory and learning in behavioral paradigms (Björklund et al., 2000; Hunter et al., 1997) and involvement of A2AR-2C in startle responses, prepulse inhibition and sensorimotor gating has been demonstrated (Sallinen et al., 1998). Disorders with deficits in these types of behavior include schizophrenia and attention deficit disorder, both considered developmental disorders.
Many studies have shown that neurotransmitters, including NE, play important roles as regulators of nervous system development (Landis, 1990; Lauder, 1993; see Lipton and Kater, 1989; Meier et al., 1991; Whitaker-Azmitia, 1991). Noradrenergic neurons in the rat differentiate by gestational day (GD) 12 and project to the cortex at GD 17 (Coyle and Molliver, 1977; Lauder and Bloom, 1974; Schlumpf et al., 1980). The timing of noradrenergic cortical innervation coincides with many important events relevant to cortical development including neurogenesis, neuronal migration, sprouting of cellular processes, and the formation of synaptic contacts. This occurs largely within the first three weeks of postnatal development in rodents, a time period in which noradrenergic innervation is established and subsequently increases to adult levels (Berger-Sweeney and Hohmann, 1997; Markus and Petit, 1987; Murrin et al., 2007).
Neonatal lesion of noradrenergic neurons produces a significant increase in synaptic density through PND 8 (Blue and Parnavelas, 1982) and an increase in cortical layer VI dendritic length (Maeda et al., 1974). Similar lesions increase the number of dendritic branches of pyramidal neurons in cortical layers III and IV (Wendlandt et al., 1977) and change dendritic orientation of layer IV neurons of the somatosensory cortex (Loeb et al., 1987). These studies indicate norepinephrine is important in regulating neuronal maturation in the CNS, particularly in cortex.
To further address the role of norepinephrine in A2AR development we employed a model of developmental norepinephrine absence, i.e., DSP4 lesions, and a model of developmental NE excess, i.e., prenatal treatment with MAM. We found that loss of NE during development has minimal effect on A2AR expression. A2AR density, agonist high affinity state and receptor functionality, as measured by linkage to G proteins, are not altered beyond the first few days by DSP4-induced loss of NE in developing rat brain. This is consistent with results in dopamine beta-hydroxylase knockout mice (DBH−/−) which have a selective and complete absence of NE across postnatal development (Sanders et al., 2006). In contrast to loss of developmental NE, increasing norepinephrine concentrations during development via MAM treatment leads to large increases in cortical A2AR.
4.1. Norepinephrine Regulation of Brain Development and A2AR
Our study employs the A2AR antagonist, [3H]RX821002 and the A2AR agonist [125I]p-iodoclonidine to measure brain A2AR. An important factor in interpreting our data is the ability of A2AR antagonists versus A2AR agonists to label different populations of the receptor. As a G-protein coupled receptor A2AR exist in agonist high and low affinity states. A2AR antagonists such [3H]RX821002 measure changes in the total receptor population (high- and low- affinity states). In contrast, ligands such as clonidine, p-iodoclonidine and [3H]-UK14304 are agonists which a have a much greater affinity for A2AR in the high-affinity state (Baron and Seigel, 1990; Gerhardt et al., 1990), and when used in very low concentrations label the high affinity state almost exclusively.
Our initial studies used the A2AR antagonist, [3H]RX821002, to examine the response of the total A2AR population to neonatal DSP4. Our results demonstrating a loss of A2AR in the first days following DSP4 lesion suggests that in neonatal cortex a large percentage of A2AR are presynaptic. Our data agree with previous studies that found a similar decrease shortly after DSP4 or 6-OHDA lesion (Dausse et al., 1982; Heal et al., 1993). Further studies used [3H]RX821002 and [125I]p-iodoclonidine to measure total A2AR density and high-affinity state, respectively. From PND 15 through PND 65 we found no difference in total or high-affinity A2AR density in frontal and parietal cortex when comparing DSP4-treated rats with controls, in agreement with others (Heal et al., 1993), whereas Dausse and colleagues found a 20% increase in A2AR in cortex from PND 45-50 rats (Dausse et al., 1982). These differences may reflect differential effects of 6-OHDA, which destroys dopaminergic as well as noradrenergic terminals, compared to DSP4, which is primarily a noradrenergic toxin, or that the earlier studies used membrane binding with [3H]clonidine as opposed to our studies which used the autoradiographic procedures with [3H]RX821002 and [125I]p-iodoclonidine. The selectivity of DSP-4 and similar findings with the specific elimination of NE in DBH−/− mice (Sanders et al., 2006), however, indicates developmental loss of norepinephrine does not produce an up-regulation of A2AR, in contrast to what is found in older animals.
Interpretation of these data requires consideration of the limitations inherent in developmental lesions. It is possible that a small fraction of a given neurotransmitter pool may sustain adequate receptor stimulation to maintain normal receptor homeostasis. The dopaminergic system, for instance, only requires 20% of endogenous dopamine in order to be functional. Our preliminary studies demonstrate that DSP-4 successfully depletes ~95% of norepinephrine from the developing cortex and hippocampus. Furthermore DBH−/− mice are completely devoid of NE postnatally and exhibit small but statistically significant decreases in [3H]RX821002 binding in the hippocampus but not in cortex (Sanders et al., 2006). From these findings we conclude it is unlikely the A2AR phenotype in our animal models is being sustained by very low levels of norepinephrine. The differences between small A2AR decreases in DBH−/− animals and no change in DSP4 lesioned neonatal rats may be due to species differences. Alternatively DBH−/− have a complete absence of norepinephrine throughout postnatal development and into adulthood, whereas our DSP4 lesions of neonates maintain less than 5% of endogenous NE (Fig. 1A). While this is a small amount of remaining neurotransmitter, it is possible that a residual amount maintains receptor stimulation and contributes to differences in A2AR phenotype between these two models.
We considered whether other neurotransmitters may be activating A2AR in the absence of norepinephrine, and therefore maintaining normal levels. It has been shown, for instance, that dopamine has an affinity for A2AR-A and A2AR-C that is comparable to norepinephrine (Zhang et al., 1999). That dopamine is activating A2AR to maintain the receptor phenotype in our studies, though, is unlikely. Dopaminergic fibers are restricted in their projection and have very sparse terminal fields in most of the cortex and hippocampus (Lindvall and Björklund, 1983). Dopamine levels in the hippocampus and occipital cortex, for example, are far lower than norepinephrine levels. Dopamine levels in the hippocampus and occipital cortex, for example, are 15 ± 2 and 14 ± 1 ng/g, respectively, whereas norepinephrine levels in the hippocampus are 582 ± 52 ng/g and in the occipital cortex 329 ± 1 (Jonsson et al., 1982). Therefore, it is unlikely that dopamine is stimulating A2AR to maintain the normal developmental phenotype in areas such as the hippocampus or cortex. Stimulation by epinephrine or serotonin is also unlikely. Although A2AR have a high affinity for epinephrine, there is very little epinephrine in the brain. While serotonergic and noradrenergic terminals broadly overlap, serotonin has a low affinity for A2AR. Therefore each of these neurotransmitters would be unlikely candidates for compensatory stimulation in NE’s absence. Based on our studies and these considerations, we conclude that the elimination of NE from the developing brain does not significantly affect A2AR expression, affinity state or linkage to G proteins.
Prior studies have documented that the MAM lesion protocol used in these studies results in a ~220% increase in the concentration of NE in cerebral cortex. Excitatory and inhibitory neurotransmitters and enzymes that are intrinsic to the projection field, such as γ-amino-butyric acid and glutamate decarboxylase, are minimally affected by MAM treatment (Johnston et al., 1981a; Johnston et al., 1979). Neurotransmitters originating from brainstem nuclei, such as serotonin and acetylcholine are increased (Johnston et al., 1981b), but it is unlikely they influence A2AR given their lack of affinity for the receptors. In addition, there are other models of increased developmental NE which show increases in A2AR. The mouse mutant coloboma, which is deficient in SNAP-25 synaptic vesicle docking protein, has increased NE in certain brain regions and parallel increased A2AR-A mRNA (Jones et al., 2001).
Treating juvenile rats with desipramine, a tricyclic antidepressant that primarily blocks norepinephrine reuptake, results in A2AR increases in prefrontal cortex (Deupree et al., 2007). Fetal cocaine, which also blocks NE reuptake, similarly results in an increase in A2AR during brain development (Booze et al., 2006; Seidler and Slotkin, 1992). The NET-KO mouse, which has increased synaptic norepinephrine levels, has a robust increase in A2AR mRNA as well as functional A2AR receptors (Gilsbach et al., 2006).
Cumulatively, these data point to a differential noradrenergic regulation of A2AR in developing versus adult brain. In general, adult animals show an increase in the A2AR high affinity state and little change in total A2AR density as a result of noradrenergic lesions during maturity. For example, 6-OHDA, which lesions noradrenergic and dopaminergic fibers, results in a doubling of [3H]clonidine high affinity binding sites in cortex (U’Prichard et al., 1979). Reserpine, which depletes the brain of monoamines including norepinephrine, results in an up-regulation of high affinity A2AR binding by A2AR agonists [3H]-UK14304 and [3H]clonidine in the cortex and other brain regions (Giralt and Garcia-Sevilla, 1989; Ribas et al., 2001). A separate study of [3H]p-aminoclonidine binding in adult rats lesioned with DSP4 found an increase in [3H]p-aminoclonidine affinity in cortex, hippocampus, striatum and cerebellum. These brain wide increases in affinity occurred in the context of an unchanged Bmax in cortex and cerebellum and slightly decreased Bmax in hippocampus and striatum (Dooley et al., 1983). An additional study found an increase in cortical A2AR, as measured by clonidine binding, 7 days after DSP4 treatment of adult rats (Wolfman 1994).
Other studies measuring A2AR with antagonists such as [3H]idazoxan or [3H]RX821002 have found little or no change in receptor density after lesioning the noradrenergic system of adults (Heal et al., 1993; Hume et al., 1992). The selective up-regulation of high affinity A2AR is consistent with our own data which shows an increase in cortical [125I]p-iodoclonidine binding when animals are lesioned with DSP4 at PND 48 and brains harvested at PND 62 (Fig. 3C). This occurs alongside no change in cortical [3H]RX821002 binding when animals are lesioned with DSP4 during a similar time frame (i.e., treated with DSP4 on PND 43 and brains harvested on PND 65; Fig. 5). This dissociation between increases in A2AR agonist binding alongside no changes in antagonist binding has been documented in other studies that deplete norepinephrine in adult rats (Ribas et al., 2001). This has also been seen in the postmortem brain of suicide victims (Callado et al., 1998; Ordway et al., 1994), consistent with a norepinephrine deficiency in severe depression.
The effects of depleting NE from the mature brain contrast with those of increasing synaptic NE by blocking its reuptake, which results in a down-regulation adrenergic receptors. This has been documented for beta-adrenergic receptors (Daws et al., 1998; Tiong and Richardson, 1990) and for A2AR using either antagonists or agonists to label the receptor (Barturen and Garcia-Sevilla, 1992; Deupree et al., 2007; Subhash et al., 2003). The response of the mature brain to alterations in NE level is the opposite of changes found in developing brain. We find a decrease or no change in total α2-AR number, high affinity state or linkage to G proteins following a developmental loss of NE and we find a robust increase in A2AR density in association with increased developmental NE.
4.2. Clinical Implications
Therapeutically, several psychotropic medications target A2AR and are used to treat psychopathology in developing as well as adult populations. Guanfacine, a partial A2AR agonist, is effective in treating children and adolescents with ADHD (Sallee et al., 2009). Mirtazapine is an antidepressant which acts as an antagonist at A2AR as well as at 5-HT2 and 5-HT3 receptors. While approved for the treatment of depression in adulthood, it also has been used to treat social phobia in children and adolescents (Mrakotsky et al., 2008). A2AR antagonist properties are also found in atypical antipsychotics used to treat adult was well as childhood psychotic and affective disorders. For example, antipsychotics such as clozapine and risperidone have affinities for A2AR that approximate or surpass their affinity for dopamine D2 receptors (Bymaster et al., 1996). Atomoxetine, a selective NE reuptake inhibitor, is also efficacious in the treatment of attention deficit hyperactivity disorder (ADHD) (Garnock-Jones and Keating, 2009). Its mechanism is thought to involve the stimulation of A2AR in prefrontal cortex secondary to increasing synaptic norepinephrine levels (Arnsten, 2009). Similarly, A2AR signaling is proposed to be important in mediating the therapeutic effects of tricyclic antidepressants (Zhang et al., 2009).
Etiologically, an increase in A2AR levels has been specifically implicated in depressed subjects who have committed suicide. Previous studies have shown that suicide victims have increased A2AR-A mRNA as well as A2AR density (De et al., 1997; Escriba et al., 2004). Polymorphisms in the A2AR-A gene have been associated with susceptibility to suicide (Fukutake et al., 2008).
Based upon the widespread clinical targeting of A2AR across the lifespan, their proposed role in mediating antidepressant action, and their implicated role in psychiatric disorders, it is important to understand the regulation of these receptors during development and how this compares to their regulation in adult brain. Our studies suggest that there may be important differences in the clinical response of developing versus adult populations to agents targeting A2AR. In particular, these findings may be especially relevant to understanding differential responses of childhood versus adult depression to antidepressants that work primarily on the noradrenergic system. Tricyclic antidepressants, monoamine oxidase inhibitors, and venlafaxine are effective in the treatment of adult depression but may not be effective in children (see Geller et al., 1999; Hazell et al., 2000; Varley, 2003; Whittington et al., 2004). Much of the clinical efficacy of these agents is attributable to their actions on the noradrenergic system (Subhash et al., 2003).
Our characterization of noradrenergic regulation across brain development sheds light on differences in adult and developing brain and suggests differential regulation of A2AR as a factor that may subserve differences in adult and juvenile depression (Murrin et al., 2007). In particular, an immature regulator system for the A2AR in the young animal may help explain the lack of efficacy of tricyclic antidepressant drugs in pediatric populations (Bylund and Reed, 2007; Deupree et al., 2007). Finally, these and other studies from this lab (Sanders et al., 2008) indicate that the long term consequences of exposure to drugs acting on A2AR differ in developing and mature brain and consideration should be given to this when treating children and adolescents with such drugs.
Research highlights.
Neonatal noradrenergic lesions with DSP4 have little effect on A2AR development.
The developmental response of A2AR to DSP4 is different from adults.
Increasing NE with MAM during development increases A2AR expression into adulthood.
The developmental change after increased NE is opposite what is expected in adults.
NE regulates A2AR development and has long term effects on A2AR function.
Acknowledgments
Role of the funding source.
This work was supported by the National Institutes of Health NS33194 (LCM) and MH64772 (DBH). The funding source (NIH) was not involved in this study in any way following study section review.
ABBREVIATIONS
- A2AR
alpha-2 adrenergic receptor
- DBH
dopamine-β-hydroxylase
- DSP4
N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride
- DTT
dithiothreitol
- GD
gestational day
- IEG
immediate early gene
- MAM
methylazoxymethanol acetate
- NE
norepinephrine
- NET
norepinephrine transporter
- PND
postnatal day (age)
- RT
room temperature
- RX821002
2-[2-(2-methoxy-1,4benzodioxanyl)]imidazoline hydrochloride
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulla FA, Smith PA. Ectopic alpha2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. J Neurosci. 1997;17:1633–1641. doi: 10.1523/JNEUROSCI.17-05-01633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Steere JC, Hunt RD. The contribution of α2-noradrenergic mechanisms to prefrontal cortical cognitive function. Arch Gen Psychiatry. 1996;53:448–455. doi: 10.1001/archpsyc.1996.01830050084013. [DOI] [PubMed] [Google Scholar]
- Baron BM, Seigel BW. p-[125I]Iodoclonidine, a novel radiolabeled agonist for studying central α2-adrenergic receptors. Molec Pharmacol. 1990;38:348–356. [PubMed] [Google Scholar]
- Barturen F, Garcia-Sevilla JA. Long term treatment with desipramine increases the turnover of alpha 2-adrenoceptors in the rat brain. Mol Pharmacol. 1992;42:846–855. [PubMed] [Google Scholar]
- Bayer SA. Quantitative 3H-thymidine radiographic analyses of neurogenesis in the rat amygdala. J Comp Neurol. 1980;194:845–875. doi: 10.1002/cne.901940409. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Hohmann CF. Behavioral consequences of abnormal cortical development: insights into developmental disabilities. Behav Brain Res. 1997;86:121–142. doi: 10.1016/s0166-4328(96)02251-6. [DOI] [PubMed] [Google Scholar]
- Björklund M, Sirviö J, Riekkinen M, Sallinen J, Scheinin M, Riekkinen P., Jr Overexpression of alpha2C-adrenoceptors impairs water maze navigation. Neuroscience. 2000;95:481–487. doi: 10.1016/s0306-4522(99)00428-5. [DOI] [PubMed] [Google Scholar]
- Blue ME, Parnavelas JG. The effect of neonatal 6-hydroxydopamine treatment on synaptogenesis in the visual cortex of the rat. J Comp Neurol. 1982;205:199–205. doi: 10.1002/cne.902050211. [DOI] [PubMed] [Google Scholar]
- Booze RM, Wallace DR, Silvers JM, Strupp BJ, Snow DM, Mactutus CF. Prenatal cocaine exposure alters alpha2 receptor expression in adolescent rats. BMC Neurosci. 2006;7:33. doi: 10.1186/1471-2202-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucheler MM, Hadamek K, Hein L. Two α2-adrenergic receptor subtypes, α2A and α2C, inhibit transmitter release in the brain of gene-targeted mice. Neuroscience. 2002;109:819–826. doi: 10.1016/s0306-4522(01)00531-0. [DOI] [PubMed] [Google Scholar]
- Bush DE, Caparosa EM, Gekker A, Ledoux J. Beta-adrenergic receptors in the lateral nucleus of the amygdala contribute to the acquisition but not the consolidation of auditory fear conditioning. Front Behav Neurosci. 2010;4:154. doi: 10.3389/fnbeh.2010.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund DB. Subtypes of α1- and α2-adrenergic receptor. FASEB J. 1992;6:832–839. doi: 10.1096/fasebj.6.3.1346768. [DOI] [PubMed] [Google Scholar]
- Bylund DB, Reed AL. Childhood and adolescent depression: why do children and adults respond differently to antidepressant drugs? Neurochem Int. 2007;51:246–253. doi: 10.1016/j.neuint.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Callado LF, Meana JJ, Grijalba B, Pazos A, Sastre M, García-Sevilla JA. Selective increase of α2A-adrenoceptor agonist binding sites in brains of depressed suicide victims. J Neurochem. 1998;70:1114–1123. doi: 10.1046/j.1471-4159.1998.70031114.x. [DOI] [PubMed] [Google Scholar]
- Cheetham SC, Viggers JA, Butler SA, Prow MR, Heal DJ. [3H]Nisoxetine - a radioligand for noradrenaline reuptake sites: correlation with inhibition of [3H]noradrenaline uptake and effect of DSP-4 lesioning and antidepressant treatments. Neuropharmacology. 1996;35:63–70. doi: 10.1016/0028-3908(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Molliver ME. Major innervation of newborn rat cortex by monoaminergic neurons. Science. 1977;196:444–447. doi: 10.1126/science.850788. [DOI] [PubMed] [Google Scholar]
- Dausse JP, Quan-Bui KHL, Meyer P. α1- and α2-Adrenoceptors in rat cerebral cortex: effects of neonatal treatment with 6-hydroxydopamine. Eur J Pharmacol. 1982;78:15–20. doi: 10.1016/0014-2999(82)90367-3. [DOI] [PubMed] [Google Scholar]
- Davis MP, Dickerson ED, Pappagallo M, Benedetti C, Grauer PA, Lycan J. Mirtazepine: heir apparent to amitriptyline? Am J Hosp Palliat Care. 2001;18:42–46. doi: 10.1177/104990910101800111. [DOI] [PubMed] [Google Scholar]
- Daws LC, Lopez R, Frazer A. Effects of antidepressant treatment on inhibitory avoidance behavior and amygdaloid beta-adrenoceptors in rats. Neuropsychopharmacology. 1998;19:300–313. doi: 10.1016/S0893-133X(98)00016-5. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Kolassa IT, Ertl V, Onyut PL, Neuner F, Elbert T, Papassotiropoulos A. A deletion variant of the α2b-adrenoceptor is related to emotional memory in Europeans and Africans. Nat Neurosci. 2007;10:1137–1139. doi: 10.1038/nn1945. [DOI] [PubMed] [Google Scholar]
- De PF, Mauger JM, Lowther S, Crompton MR, Katona CL, Horton RW. Brain alpha-adrenoceptors in depressed suicides. Brain Res. 1997;757:60–68. doi: 10.1016/s0006-8993(97)00138-8. [DOI] [PubMed] [Google Scholar]
- Deupree JD, Reed AL, Bylund D. Differential effects of the tricyclic antidepressant, desipramine, on the density of adrenergic receptors in juvenile and adult rats. J Pharmacol Exp Ther. 2007;321:770–776. doi: 10.1124/jpet.106.118935. [DOI] [PubMed] [Google Scholar]
- Dooley DJ, Bittiger H, Hauser KL, Bischoff SF, Waldmeier PC. Alteration of central alpha2- and beta-adrenergic receptors in the rat after DSP-4, a selective noradrenergic neurotoxin. Neuroscience. 1983;9:889–898. doi: 10.1016/0306-4522(83)90277-4. [DOI] [PubMed] [Google Scholar]
- Duman RS, Enna SJ. A procedure for measuring α2-adrenergic receptor-mediated inhibition of cyclic AMP accumulation in rat brain slices. Brain Res. 1986;384:391–394. doi: 10.1016/0006-8993(86)91179-0. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Increased mRNA expression of alpha2A-adrenoceptors, serotonin receptors and mu-opioid receptors in the brains of suicide victims. Neuropsychopharmacology. 2004;29:1512–1521. doi: 10.1038/sj.npp.1300459. [DOI] [PubMed] [Google Scholar]
- Felten DL, Hallman H, Jonsson G. Evidence for a neurotrophic role of noradrenaline neurons in the postnatal development of rat cerebral cortex. J Neurocytol. 1982;11:119–135. doi: 10.1007/BF01258008. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AFT. Treatment with the noradrenergic alpha-2 agonist clonidine, but not diazepam, improves spatial working memory in normal young Rhesus monkeys. Neuropsychopharmacology. 1999;21:611–621. doi: 10.1016/S0893-133X(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Kessler LE, Borja CM, Kobilka BK, Limbird LE, Arnsten AF. Mutation of the alpha2A-adrenoceptor impairs working memory performance and annuls cognitive enhancement by guanfacine. J Neurosci. 2002;22:8771–8777. doi: 10.1523/JNEUROSCI.22-19-08771.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukutake M, Hishimoto A, Nishiguchi N, Nushida H, Ueno Y, Shirakawa O, Maeda K. Association of alpha2A-adrenergic receptor gene polymorphism with susceptibility to suicide in Japanese females. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1428–1433. doi: 10.1016/j.pnpbp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Garnock-Jones KP, Keating GM. Atomoxetine: a review of its use in attention-deficit hyperactivity disorder in children and adolescents. Paediatr Drugs. 2009;11:203–226. doi: 10.2165/00148581-200911030-00005. [DOI] [PubMed] [Google Scholar]
- Geller B, Reising D, Leonard HL, Riddle MA, Walsh BT. Critical review of tricyclic antidepressant use in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38:513–516. doi: 10.1097/00004583-199905000-00012. [DOI] [PubMed] [Google Scholar]
- Gerhardt MA, Wade SM, Neubig RR. p-[125I]iodoclonidine is a partial agonist at the alpha 2-adrenergic receptor. Mol Pharmacol. 1990;38:214–221. [PubMed] [Google Scholar]
- Gilsbach R, Faron-Gorecka A, Rogoz Z, Bruss M, Caron MG, Dziedzicka-Wasylewska M, Bonisch H. Norepinephrine transporter knockout-induced up-regulation of brain alpha2A/C-adrenergic receptors. J Neurochem. 2006;96:1111–1120. doi: 10.1111/j.1471-4159.2005.03598.x. [DOI] [PubMed] [Google Scholar]
- Giralt MT, Garcia-Sevilla JA. Acute and long-term regulation of brain alpha 2-adrenoceptors after manipulation of noradrenergic transmission in the rat. Eur J Pharmacol. 1989;164:455–466. doi: 10.1016/0014-2999(89)90253-7. [DOI] [PubMed] [Google Scholar]
- Happe HK, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor-mediated [35S]GTPγS binding in rat brain: an autoradiographic study. FASEB J. 1999;13:A798. [Google Scholar]
- Happe HK, Coulter CL, Gerety ME, Sanders JD, O’Rourke M, Bylund DB, Murrin LC. Alpha-2 adrenergic receptor development in rat CNS: an autoradiographic study. Neuroscience. 2004;123:167–178. doi: 10.1016/j.neuroscience.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Hazell P, O’Connell D, Heathcote D, Henry D. Tricyclic drugs for depression in children and adolescents. Cochrane Database Syst Rev. 2000:CD002317. doi: 10.1002/14651858.CD002317. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Butler SA, Prow MR, Buckett WR. Quantification of presynaptic alpha 2-adrenoceptors in rat brain after short-term DSP-4 lesioning. Eur J Pharmacol. 1993;249:37–41. doi: 10.1016/0014-2999(93)90659-6. [DOI] [PubMed] [Google Scholar]
- Hein L, Altman JD, Kobilka BK. Two functionally distinct α2-adrenergic receptors regulate sympathetic neurotransmission. Nature. 1999;402:181–184. doi: 10.1038/46040. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Guyenet PG. α2-Adrenergic autoreceptors in A5 and A6 neurons of neonate rats. Am J Physiol. 1997;273:H2290–H2295. doi: 10.1152/ajpheart.1997.273.5.H2290. [DOI] [PubMed] [Google Scholar]
- Hume SP, Lammertsma AA, Opacka-Juffry J, Ahier RG, Myers R, Cremer JE, Hudson AL, Nutt DJ, Pike VW. Quantification of in vivo binding of [3H]RX 821002 in rat brain: evaluation as a radioligand for central alpha 2-adrenoceptors. Int J Rad Appl Instrum B. 1992;19:841–849. doi: 10.1016/0883-2897(92)90170-4. [DOI] [PubMed] [Google Scholar]
- Hunter JC, Fontana DJ, Hedley LR, Jasper JR, Lewis R, Link RE, Secchi R, Sutton J, Eglen RM. Assessment of the role of α2-adrenoceptor subtypes in the antinociceptive, sedative and hypothermic action of dexmedetomidine in transgenic mice. Br J Pharmacol. 1997;122:1339–1344. doi: 10.1038/sj.bjp.0701520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaim-Etcheverry G, Zieher LM. DSP-4: a novel compound with neurotoxic effects on noradrenergic neurons of adult and developing rats. Brain Res. 1980;188:513–523. doi: 10.1016/0006-8993(80)90049-9. [DOI] [PubMed] [Google Scholar]
- Jeong SW, Ikeda SR. Effect of G protein heterotrimer composition on coupling of neurotransmitter receptors to N-type Ca(2+) channel modulation in sympathetic neurons. Proc Natl Acad Sci U S A. 2000;97:907–912. doi: 10.1073/pnas.97.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Carman AB, Coyle JT. Effects of fetal treatment with methylazoxymethanol acetate at various gestational dates on the neurochemistry of the adult neocortex of the rat. J Neurochem. 1981a;36:124–128. doi: 10.1111/j.1471-4159.1981.tb02386.x. [DOI] [PubMed] [Google Scholar]
- Johnston MV, Grzanna R, Coyle JT. Methylazoxymethanol treatment of fetal rats results in abnormally dense noradrenergic innervation of neocortex. Science. 1979;203:369–371. doi: 10.1126/science.32620. [DOI] [PubMed] [Google Scholar]
- Johnston MV, McKinney M, Coyle JT. Neocortical cholinergic innervation: a description of extrinsic and intrinsic components in the rat. Exp Brain Res. 1981b;43:159–172. doi: 10.1007/BF00237760. [DOI] [PubMed] [Google Scholar]
- Jolkkonen J, Puurunen K, Koistinaho J, Kauppinen R, Haapalinna A, Nieminen L, Sivenius J. Neuroprotection by the alpha2-adrenoceptor agonist, dexmedetomidine, in rat focal cerebral ischemia. Eur J Pharmacol. 1999;372:31–36. doi: 10.1016/s0014-2999(99)00186-7. [DOI] [PubMed] [Google Scholar]
- Jones MD, Williams ME, Hess EJ. Expression of catecholaminergic mRNAs in the hyperactive mouse mutant coloboma. Brain Res Mol Brain Res. 2001;96:114–121. doi: 10.1016/s0169-328x(01)00281-9. [DOI] [PubMed] [Google Scholar]
- Jonsson G, Hallman H, Sundstrom E. Effects of noradrenaline neurotoxin DSP4 on the postnatal development of central noradrenaline neurons in the rat. Neuroscience. 1982;7:2895–2907. doi: 10.1016/0306-4522(82)90112-9. [DOI] [PubMed] [Google Scholar]
- Kable JW, Murrin LC, Bylund DB. In vivo gene modification elucidates subtype-specific functions of α2-adrenergic receptors. J Pharmacol Exp Ther. 2000;293:1–7. [PubMed] [Google Scholar]
- Kamisaki Y, Hamahashi T, Hamada T, Maeda K, Itoh T. Presynaptic inhibition by clonidine of neurotransmitter amino acid release in various brain regions. Eur J Pharmacol. 1992;217:57–63. doi: 10.1016/0014-2999(92)90511-2. [DOI] [PubMed] [Google Scholar]
- Landis SC. Target regulation of neurotransmitter phenotype. Trends Neurosci. 1990;8:344–350. doi: 10.1016/0166-2236(90)90147-3. [DOI] [PubMed] [Google Scholar]
- Lauder JM. Neurotransmitters as growth regulatory signals: role of receptors and second messengers. Trends Neurosci. 1993;16:233–240. doi: 10.1016/0166-2236(93)90162-f. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Bloom FE. Ontogeny of monoamine neurons in the locus coeruleus, raphe nuclei and substantia nigra of the rat. I. Cell differentiation. J Comp Neurol. 1974;155:469–481. doi: 10.1002/cne.901550407. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Rakic P. Unique profiles of the α1-, α2-, and β-adrenergic receptors in the developing cortical plate and transient embryonic zones of the rhesus monkey. J Neurosci. 1994;14:4064–4078. doi: 10.1523/JNEUROSCI.14-07-04064.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Björklund A. Dopamine- and norepinephrine-containing neuron systems: their anatomy in the rat brain. In: Emson PC, editor. Chemical Neuroanatomy. Raven Press; New York: 1983. pp. 229–255. [Google Scholar]
- Lipton SA, Kater SB. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989;12:265–270. doi: 10.1016/0166-2236(89)90026-x. [DOI] [PubMed] [Google Scholar]
- Loeb EP, Chang FF, Greenough WT. Effects of neonatal 6-hydroxydopamine treatment upon morphological organization of the posteromedial barrel subfield in mouse somatosensory cortex. Brain Res. 1987;403:113–120. doi: 10.1016/0006-8993(87)90129-6. [DOI] [PubMed] [Google Scholar]
- Luhmann HJ, Hanganu I, Kilb W. Cellular physiology of the neonatal rat cerebral cortex. Brain Res Bull. 2003;60:345–353. doi: 10.1016/s0361-9230(03)00059-5. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting - homing in on α2-adrenoceptor-subtype function. Trends Pharmacol Sci. 1997;18:211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Maeda T, Tohyama M, Shimizu N. Modification of postnatal development of neocortex in rat brain with experimental deprivation of locus coeruleus. Brain Res. 1974;70:515–520. doi: 10.1016/0006-8993(74)90261-3. [DOI] [PubMed] [Google Scholar]
- Markus EJ, Petit TL. Neocortical synaptogenesis, aging, and behavior: lifespan development in the motor-sensory system of the rat. Exp Neurol. 1987;96:262–278. doi: 10.1016/0014-4886(87)90045-8. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Zornow MH, Rabin BC, Maze M. The alpha 2 adrenergic agonist, dexmedetomidine, selectively attenuates ischemia-induced increases in striatal norepinephrine concentrations. Brain Res. 1993;627:325–329. doi: 10.1016/0006-8993(93)90337-m. [DOI] [PubMed] [Google Scholar]
- McCune SK, Hill JM. Ontogenic expression of two alpha-1 adrenergic receptor subtypes in the rat brain. J Mol Neurosci. 1995;6:51–62. doi: 10.1007/BF02736759. [DOI] [PubMed] [Google Scholar]
- Meier E, Hertz L, Schousboe A. Neurotransmitters as developmental signals. Neurochem Int. 1991;19:1–15. [Google Scholar]
- Miller JA, Hoffer BJ, Zahniser NR. An improved calibration procedure for computer-based quantitative autoradiography utilizing a mathematical model for the non-linear response of camera and film. J Neurosci Methods. 1988;22:233–238. doi: 10.1016/0165-0270(88)90044-1. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Mrakotsky C, Masek B, Biederman J, Raches D, Hsin O, Forbes P, de MC, DeMaso DR, Gonzalez-Heydrich J. Prospective open-label pilot trial of mirtazapine in children and adolescents with social phobia. J Anxiety Disord. 2008;22:88–97. doi: 10.1016/j.janxdis.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Schutsky K, Jin SH, Thomas SA. Norepinephrine and (1)-adrenergic signaling facilitate activation of hippocampal CA1 pyramidal neurons during contextual memory retrieval. Neuroscience. 2011;181:109–116. doi: 10.1016/j.neuroscience.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrin LC, Gerety ME, Happe HK, Bylund DB. Inverse agonism at α2-adrenoceptors in native tissue. Eur J Pharmacol. 2000;398:185–191. doi: 10.1016/s0014-2999(00)00317-4. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone V, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Widdowson PS, Smith KS, Halaris A. Agonist binding to α2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. J Neurochem. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- Parnavelas JG, Blue ME. The role of the noradrenergic system on the formation of synapses in the visual cortex of the rat. Developmental Brain Research. 1982;3:140–144. doi: 10.1016/0165-3806(82)90082-7. [DOI] [PubMed] [Google Scholar]
- Patel KP, Ciriello J, Kline RL. Noradrenergic mechanisms in brain and peripheral organs after aortic nerve transection. Am J Physiol. 1981;240:H481–H486. doi: 10.1152/ajpheart.1981.240.4.H481. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas C, Miralles A, Busquets X, Garcia-Sevilla JA. Brain α2-adrenoceptors in monoamine-depleted rats: increased receptor density, G coupling proteins, receptor turnover and receptor mRNA. Br J Pharmacol. 2001;132:1467–1476. doi: 10.1038/sj.bjp.0703963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallee FR, Lyne A, Wigal T, McGough JJ. Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2009;19:215–226. doi: 10.1089/cap.2008.0080. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M. Adrenergic α2C-receptors modulate the acoustic startle reflex, prepulse inhibition, and aggression in mice. J Neurosci. 1998;18:3035–3042. doi: 10.1523/JNEUROSCI.18-08-03035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Development of the norepinephrine transporter in the rat CNS. Neuroscience. 2005a;130:107–117. doi: 10.1016/j.neuroscience.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Bylund DB, Murrin LC. Differential effects of neonatal norepinephrine lesions on immediate early gene expression in developing and adult rat brain. Neuroscience. 2008;157:821–832. doi: 10.1016/j.neuroscience.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JD, Happe HK, Murrin LC. A transient expression of functional alpha2-adrenergic receptors in white matter of the developing brain. Synapse. 2005b;57:213–222. doi: 10.1002/syn.20174. [DOI] [PubMed] [Google Scholar]
- Sanders JD, Szot P, Weinshenker D, Happe HK, Bylund DB, Murrin LC. Analysis of brain adrenergic receptors in dopamine-beta-hydroxylase knockout mice. Brain Res. 2006;1109:45–53. doi: 10.1016/j.brainres.2006.06.033. [DOI] [PubMed] [Google Scholar]
- Schlumpf M, Lichtensteiger W, Shoemaker WJ, Bloom FE. Fetal monoamine systems: early stages and cortical projections. In: Parvez H, Parvez S, editors. Biogenic Amines in Development. Elsevier/North Holland; Amsterdam: 1980. pp. 567–590. [Google Scholar]
- Seidler FJ, Slotkin TA. Fetal cocaine exposure causes persistent noradrenergic hyperactivity in rat brain regions: effects on neurotransmitter turnover and receptors. J Pharmacol Exp Ther. 1992;263:413–421. [PubMed] [Google Scholar]
- Shen P, Gundlach AL. Differential modulatory effects of alpha- and beta-adrenoceptor agonists and antagonists on cortical immediate-early gene expression following focal cerebrocortical lesion-induced spreading depression. Mol Brain Res. 2000;83:133–144. doi: 10.1016/s0169-328x(00)00216-3. [DOI] [PubMed] [Google Scholar]
- Shen PJ, Burazin TCD, Gundlach AL. Noradrenergic regulation of immediate early gene expression in rat forebrain: differential effects of α1- and α2-adrenoceptor drugs. Mol Brain Res. 1995;28:222–230. doi: 10.1016/0169-328x(94)00208-v. [DOI] [PubMed] [Google Scholar]
- Stone EA, Zhang Y, John S, Filer JD, Bing G. Effect of locus coeruleus lesion on c-fos expression in the cerebral cortex caused by yohimbine injection or stress. Brain Res. 1993;603:181–185. doi: 10.1016/0006-8993(93)91236-l. [DOI] [PubMed] [Google Scholar]
- Subhash MN, Nagaraja MR, Sharada S, Vinod KY. Cortical alpha-adrenoceptor downregulation by tricyclic antidepressants in the rat brain. Neurochem Int. 2003;43:603–609. doi: 10.1016/s0197-0186(03)00097-4. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejani-Butt SM. [3H]Nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther. 1992;260:427–436. [PubMed] [Google Scholar]
- Tiong AH, Richardson JS. Beta-adrenoceptor and post-receptor components show different rates of desensitization to desipramine. Eur J Pharmacol. 1990;188:411–415. doi: 10.1016/0922-4106(90)90203-a. [DOI] [PubMed] [Google Scholar]
- U’Prichard DC, Bechtel WD, Rouot BM, Snyder SH. Multiple apparent alpha-noradrenergic receptor binding sites in rat brain: effect of 6-hydroxydopamine. Mol Pharmacol. 1979;16:47–60. [PubMed] [Google Scholar]
- Unnerstall JR, Kopajtic TA, Kuhar MJ. Distribution of α2 agonist binding sites in the rat and human central nervous system: analysis of some functional, anatomic correlates of the pharmacologic effects of clonidine and related adrenergic agents. Brain Research Reviews. 1984;7:69–101. doi: 10.1016/0165-0173(84)90030-4. [DOI] [PubMed] [Google Scholar]
- Varley CK. Psychopharmacological treatment of major depressive disorder in children and adolescents. JAMA. 2003;290:1091–1093. doi: 10.1001/jama.290.8.1091. [DOI] [PubMed] [Google Scholar]
- Wade SM, Lan K, Moore DJ, Neubig RR. Inverse agonist activity at the α2A-adrenergic receptor. Molec Pharmacol. 2001;59:532–542. doi: 10.1124/mol.59.3.532. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Alburges ME, Hunt MAE, Bylund DB. Differential localization of α2-adrenergic receptor subtypes in brain. Pharmacol Biochem Behav. 1992;41:267–273. doi: 10.1016/0091-3057(92)90097-y. [DOI] [PubMed] [Google Scholar]
- Wendlandt S, Crow TJ, Stirling RV. The involvement of the noradrenergic system arising from the locus coeruleus in the postnatal development of the cortex in rat brain. Brain Res. 1977;125:1–9. doi: 10.1016/0006-8993(77)90355-9. [DOI] [PubMed] [Google Scholar]
- Whitaker-Azmitia PM. Role of serotonin and other neurotransmitter receptors in brain development: basis for developmental pharmacology. Pharmacol Rev. 1991;43:553–561. [PubMed] [Google Scholar]
- Whittington CJ, Kendall T, Fonagy P, Cottrell D, Cotgrove A, Boddington E. Selective serotonin reuptake inhibitors in childhood depression: systematic review of published versus unpublished data. Lancet. 2004;363:1341–1345. doi: 10.1016/S0140-6736(04)16043-1. [DOI] [PubMed] [Google Scholar]
- Williams NG, Zhong H, Minneman KP. Differential coupling of α1-, α2-, and β-adrenergic receptors to mitogen-activated protein kinase pathways and differentiation in transfected PC12 cells. J Biol Chem. 1998;273:24624–24632. doi: 10.1074/jbc.273.38.24624. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Leslie FM. α2B Adrenoceptor mRNA expression during rat brain development. Developmental Brain Research. 1997;100:90–100. doi: 10.1016/s0165-3806(97)00035-7. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoceptors during rat brain development - I. α2A messenger RNA expression. Neuroscience. 1997a;76:241–260. doi: 10.1016/s0306-4522(96)00368-5. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoceptors during rat brain development - II. α2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1997b;76:261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Wolfman C, Abo V, Calvo D, Medina J, Dajas F, Silveira R. Recovery of central noradrenergic neurons one year after the administration of the neurotoxin DSP4. Neurochem Int. 1994;25:395–400. doi: 10.1016/0197-0186(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Wong DT, Bymaster FP. Effect of nisoxetine on uptake of catecholamines in synaptosomes isolated from discrete regions of rat brain. Biochem Pharmacol. 1976;25:1979–1983. doi: 10.1016/0006-2952(76)90053-8. [DOI] [PubMed] [Google Scholar]
- Wong DT, Threlkeld PG, Best KL, Bymaster FP. A new inhibitor of norepinephrine uptake devoid of affinity for receptors in rat brain. J Pharmacol Exp Ther. 1982;222:61–65. [PubMed] [Google Scholar]
- Woodcock EA, Johnston CI. Characterization of adenylate cyclase-coupled alpha2-adrenergic receptors in rat renal cortex using [3H]yohimbine. Molec Pharmacol. 1982;22:589–594. [PubMed] [Google Scholar]
- Yamazato M, Sakima A, Nakazato J, Sesoko S, Muratani H, Fukiyama K. Hypotensive and sedative effects of clonidine injected into the rostral ventrolateral medulla of conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1868–R1876. doi: 10.1152/ajpregu.2001.281.6.R1868. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Whisler LR, Huang Y, Xiang Y, O’Donnell JM. Postsynaptic α-2 adrenergic receptors are critical for the antidepressant-like effects of desipramine on behavior. Neuropsychopharmacology. 2009;34:1067–1077. doi: 10.1038/npp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Klimek V, Farley JT, Zhu M-Y, Ordway GA. α2C Adrenoceptors inhibit adenylyl cyclase in mouse striatum: potential activation by dopamine. J Pharmacol Exp Ther. 1999;289:1286–1292. [PubMed] [Google Scholar]