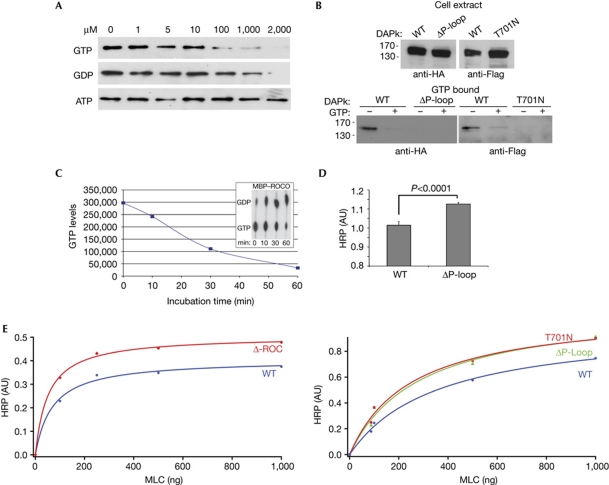
Figure 2.
GTP binds to the P-loop and attenuates DAPk catalytic activity. (A) Cell lysates expressing Flag–DAPk WT were incubated with GTP-agarose beads. After incubation in the presence of increasing concentrations of free GTP, GDP or ATP, bound proteins were subjected to western blot analysis with Flag antibody. (B) Cell lysates expressing HA–DAPk WT or ΔP-loop (left) or Flag–DAPk WT or T701N (right) were incubated with GTP-agarose beads. After incubation in the presence or absence of free GTP (2 mM), bound proteins were subjected to western blot analysis with HA or Flag antibodies. (C) MBP–ROCO was assayed for GTPase activity in the presence of [α-32P]GTP. Aliquots representing the indicated time points were resolved by thin layer chromatography (inset). The graph represents the loss of [α-32P]GTP along the course of the reaction. (D,E) The catalytic activity of DAPk was assessed by an ELISA assay optimized to detect the phosphorylation of the DAPk substrate MLC on Ser 19. (D) Mean activity±s.d. (n=4) of DAPk WT and ΔP-loop towards non-limiting amounts of MLC (1 μg) on incubation for 3 min, P<0.0001, Student's t-test. (E) The catalytic activity of ΔROC (left) or ΔP-loop and T701N mutant (right) were compared with that of DAPk WT in a Michaelis–Menten plot and the curves were fitted using the method of the least squares. ATP, adenosine triphosphate; AU, arbitrary units; DAPk, death-associated protein kinase; HA, haemagglutinin; HRP, horseradish peroxidase; MBP, maltose-binding protein; ROC, Ras of complex proteins; WT, wild type.