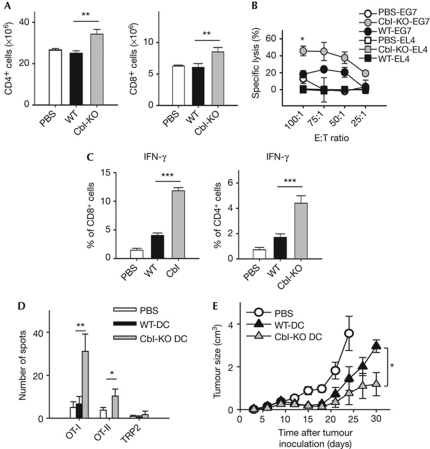
Figure 2.
c-Cbl-knockout BMDCs have enhanced pro-Th1 immunogenicity in vivo. (A) Naive, 5–6-week-old mice were subjected to footpad vaccination with PBS (n=5), WT (n=12) or Cbl-KO BMDCs (n=12, 2 × 106 cell/mouse) for 2 weeks before being killed. BMDCs were treated with Ova and stimulated with LPS (1 μg/ml) before vaccination. Splenocytes were counted, and percentages of CD8+ cells were quantified by FACS analysis (n=3; **P<0.01). (B) Splenocytes (effector cells) isolated from mice described in (A) were cultured with either the parental EL4 or Ova-expressing E.G7-Ova cells (target cells) at the indicated ratios. Target cells were pre-labelled with 51Cr for quantification of lysis caused by cell-mediated cytotoxicity. Percentage lysis was calculated by normalization against samples lysed with Triton X-100. Data were in triplicate and representative of samples derived from six pairs of WT and Cbl-KO mice (for control group, n=3; *P<0.05). (C) Splenocytes isolated from vaccinated mice described in (A) were stimulated with either OT-I (left panel, n=6) or OT-II peptide (right panel, n=6) overnight before intracellular staining of murine IFN-γ (***P<0.001). (D) Splenocytes from vaccinated mice of the groups described in (A) were subjected to ELISpot analysis for quantification of IFN-γ+ cells 5 weeks post-vaccination (n=9; *P<0.05). Cells were stimulated with indicated peptides overnight before assay. (E) E.G7-Ova tumour-bearing mice that were 5–6 weeks old were vaccinated with the indicated dendritic cell vaccines as described in (A), and subjected to tumour measurements every 3 days (n=5). Before vaccination, BMDCs were treated as described in (A). *P<0.05. Mice bearing tumours larger than 3 cm3 were killed. All data represent mean±s.e. Cbl-KO, c-Cbl-knockout; DC, dendritic cell; IFN, interferon; LPS, lipopolysaccharide; Ova, ovalbumin; TRP2, tyrosinase-related protein 2; WT, wild type.