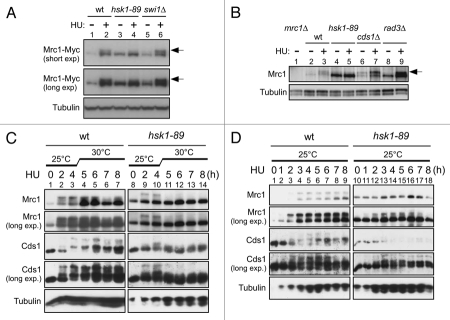
Figure 1.
HU-induced hyperphosphorylation of Mrc1 is impaired in hsk1-89. (A and B) The whole cell extracts prepared from the indicated cells were analyzed by western blotting using anti-Myc (A), anti-Mrc1 (B) and anti-α-tubulin (A and B) antibodies. After addition of 15 mM HU (A) or 25 mM HU (B) to the cultures grown at 25°C, the cells were kept growing at 25°C for 4 hrs (A) or at 30°C (non-permissive for hsk1-89) for 3 hrs (B). Arrows indicate phosphorylated forms of Mrc1 protein. (A) Lanes 1 and 2, KT2791; lanes 3 and 4, MS346; lanes 5 and 6, MS401. (B) Lane 1, MS252; lanes 2 and 3, YM71; lanes 4 and 5, KO147; lanes 7 and 8, NI485. Slight increase of hyperphosphorylation of Mrc1 in swi1Δ cells may be due to increased fork damages in this strain. (C and D) The whole cell extracts prepared from YM71 (hsk1+, lanes 1–7) and KO147 (hsk1-89, lanes 8–14) were analyzed by western blotting using antibodies indicated. After addition of 15 mM HU to the cells grown at 25°C, they were shifted to 30°C for 4 hrs (C) or kept at 25°C throughout the experiment (D). In (C and D), the samples were analyzed on 7.5% SDS-PAGE (polyacrylamide:bisacrylamide = 99:1) containing 25 µM Phos-tag (1st, 2nd and 5th parts) or on 6.5% SDS-PAGE (99:1) containing 25 µM Phos-tag (3rd and 4th parts).