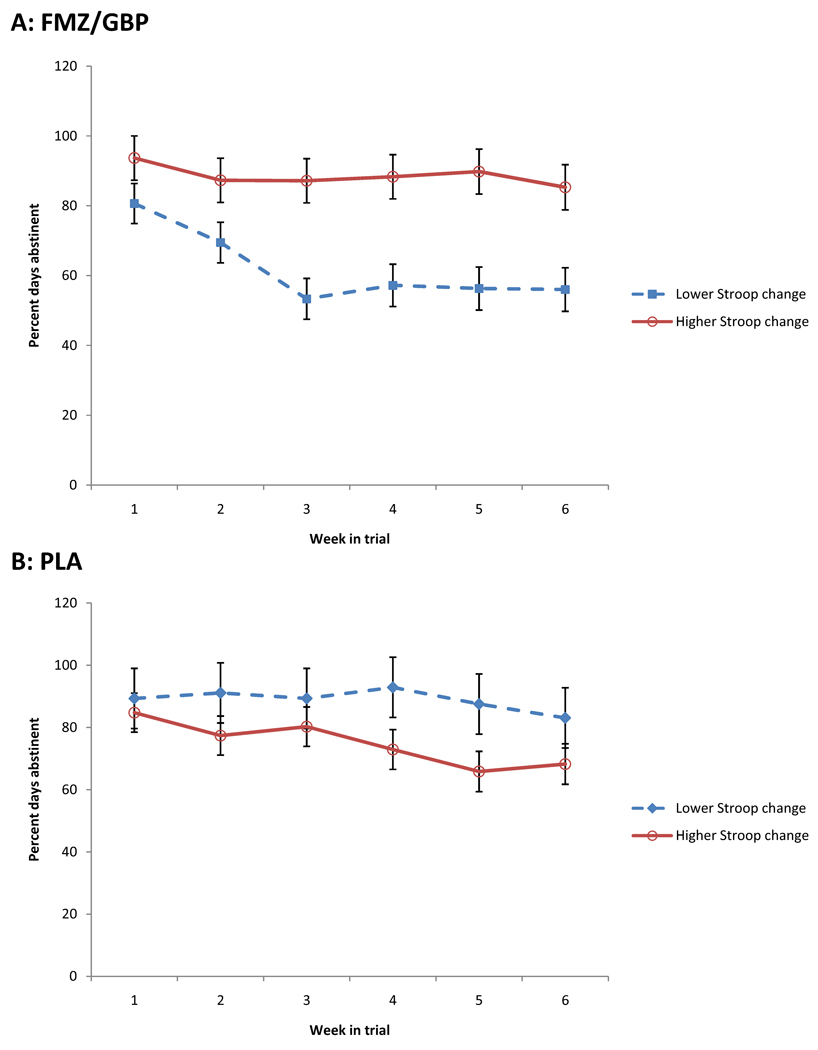
Figure 2.
Percent days abstinent during the 6-week treatment trial for participants treated with flumazenil and gabapentin (A: FMZ/GBP) and with placebos (B: PLA). Participants who were treated with active medications who had greater than average improvement on the Stroop interference trial between Days 3 and 7 had more abstinence throughout the trial, while the opposite was true for participants treated with placebos. Figures are least-squares means (± standard errors) from the linear mixed model.