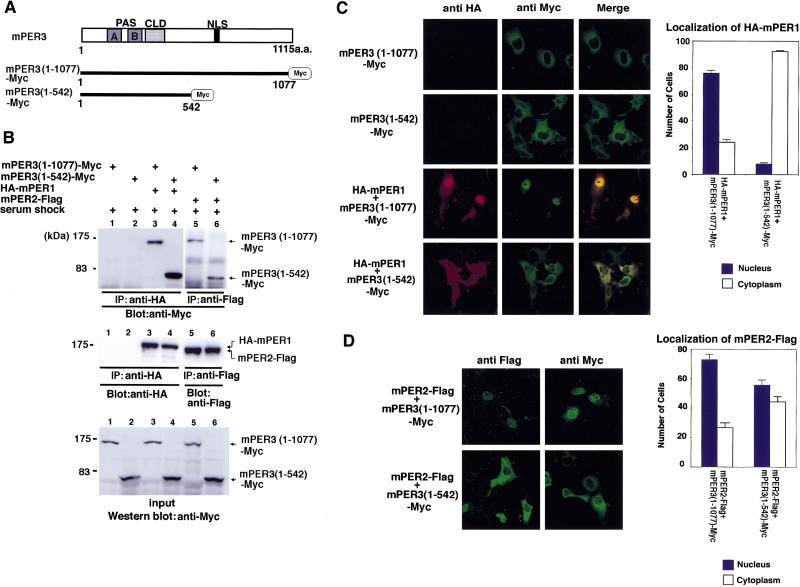
Figure 4.
Nuclear entry of a truncation mutant of mPER3 protein lacking the carboxy-terminal half containing the NLS. (A) Schematic diagrams of two mPER3-Myc constructs. (B) Immunoprecipitation showing that both mPER3 (1–1077)-Myc and mPER3 (1–542)-Myc associate with mPER1 and mPER2 protein. (C) Double-label immunofluorescence of cells coexpressing mPER3 (1–1077)-Myc and HA-mPER1, or mPER3 (1–542)-Myc and HA-mPER1. Although mPER1 coexpressed with mPER3 (1–1077) showed nuclear localization, truncated mPER3 (1–542)-Myc failed to allow nuclear entry of coexpressed mPER1 protein. Expression of only mPER3 (1–1077)-Myc or mPER3 (1–542)-Myc in COS7 cells revealed positive cytoplasmic staining to anti-Myc and no immunoreactivity against anti-HA antisera. Serum shock was performed in every case. Cell counts shown at right represent the mean of three independent experiments, and error bars indicate the s.e.m. One-hundred stained cells were observed and counted in each experiment. (D) Immunofluorescence of mPER3 (1–542)-Myc coexpressed with mPER2. mPER3 (1–542)-Myc also failed to promote nuclear entry of mPER2, but its effect on mPER2 was much weaker than that seen for mPER1. As above, serum shock was performed for all experiments. Results of cell counts represent the mean of three independent experiments, and error bars indicate the s.e.m. One-hundred stained cells were observed and counted in each experiment.