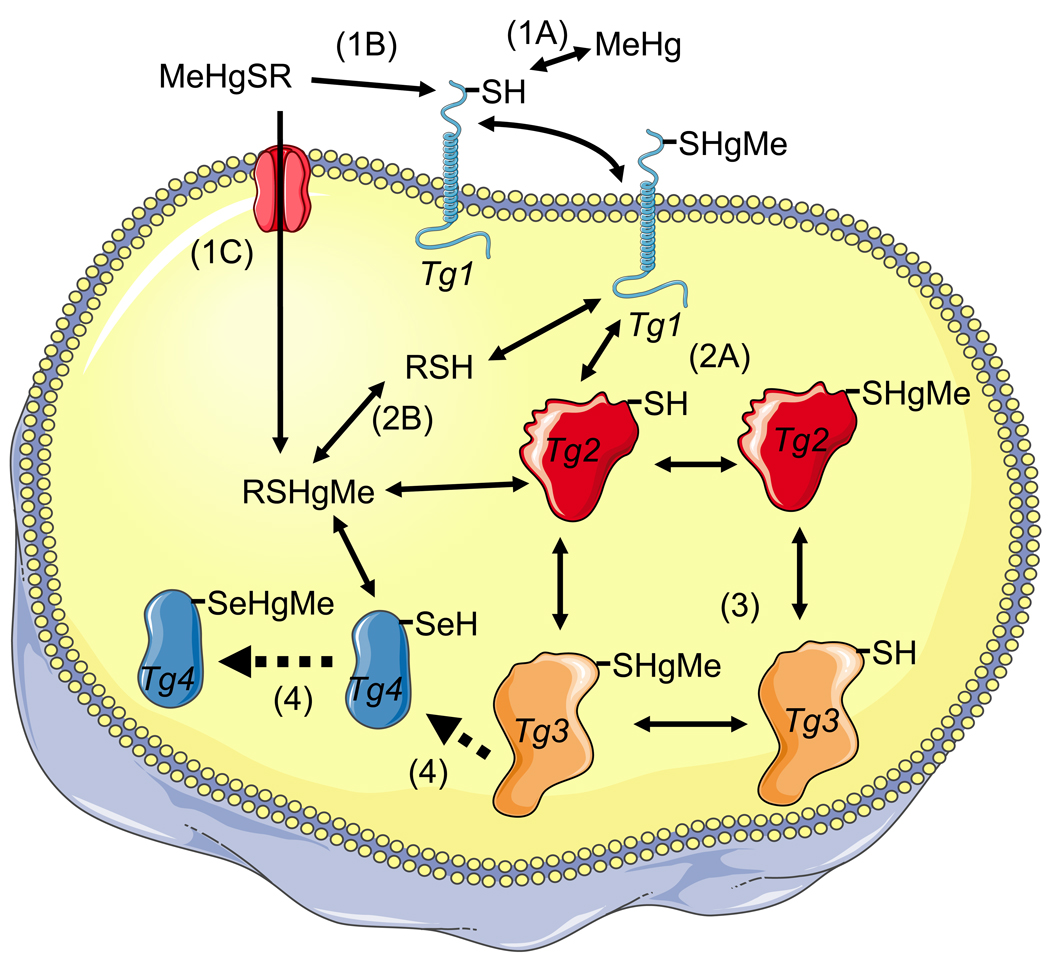
Figure 1. Interaction of MeHg or RSHgMe (a complex of MeHg with a low weight molecular thiol, e.g., cysteine or glutathione) with target cell proteins via exchange reactions.
The first event can be of three types: A) MeHg (free MeHg) can react with a target protein (Tg 1) to oxidize it; B) the low-molecular weight complex of MeHg with a thiol-containing molecule (cysteine or small peptides derived from MeHg contaminated fish muscle proteins) can participate in an exchange reaction with the target protein 1 (Tg 1), oxidizing the Tg 1 and regenerating the free thiol molecule; and C) the complex MeHg-cysteine (MeHgSR) can be transported as a mimetic of methionine. The second event can be of two types: A) the MeHg bound to target protein 1 (Tg 1-HgMe complex) can participate in an exchange reaction with a second target protein (Tg 2) to oxidize it and release the free Tg 1; or B) the exchange can occur with intracellular low-molecular- weight thiols (e.g., cysteine or GSH) to form the MeHgSG or MeHgSCys complex and release the free Tg 1. The third event is similar to that described in the second event (i.e., an exchange reaction between a target protein with a different target protein or with intracellular low-molecular thiols). The fourth event represents the tentative unidirectional (indicated by the broken arrows) reactions of intracellular low-molecular MeHg-thiol complexes (RSHgMe) or of a target protein (Tg 3) with a target selenoprotein (Tg 4). In these cases, the selenocysteyl residue of the Tg 4 selenoprotein is oxidized by MeHg and hypothetically cannot participate in an exchange reaction due to the higher affinity of a selenol group for MeHg than that of a thiol group. The aforementioned events (1–4) are represented as numbers (or numbers plus letters) in parentheses (e.g., 1A).