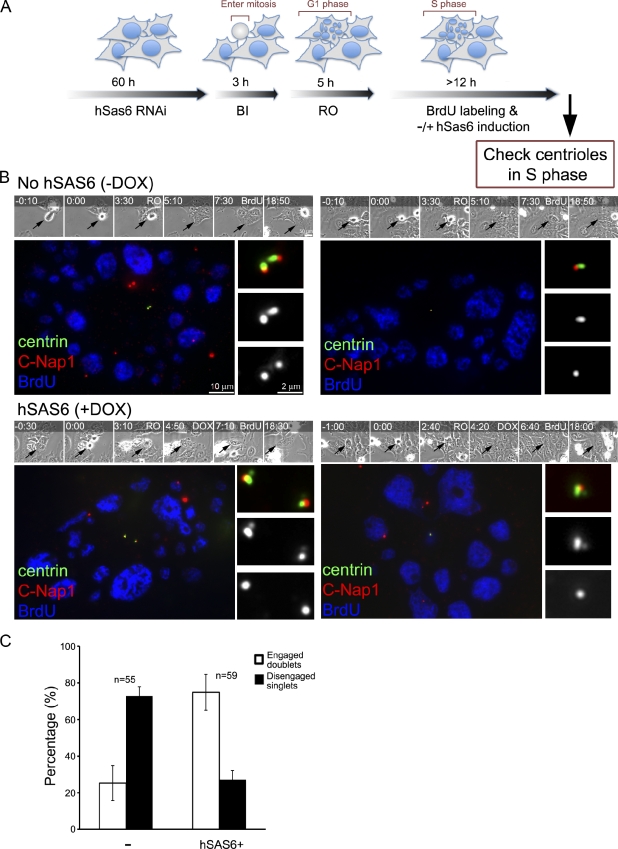
Figure 6.
MTOC-competent centrioles, when disengaged, can duplicate normally without Plk1. (A) Experimental scheme. To generate freestanding mother centrioles, asynchronously proliferating cells stably transfected with constructs that direct the expression of an RNAi-resistant form of hSas-6 (hSas-6R) from a tetracycline-inducible promoter were depleted of endogenous hSas-6 by RNAi. The hSas-6–depleted cells were filmed by time-lapse microscopy and then treated with a Plk1 inhibitor (BI-2536; BI) during mitotic entry followed by a Cdk1 inhibitor (RO-3306; RO), which induce mitotic exit. 5 h after mitotic exit, cells were allowed to progress to S/G2 phase (marked by BrdU labeling) for another 12 h, during which cells were either maintained as hSas-6 depleted or treated with doxycycline (DOX) to induce hSas-6R expression. (B) Representing images of cells in S/G2 phase had gone through the experimental scheme described in Fig. 3 A and been treated with (+) or without (–) doxycycline. Manipulated cells (arrows) were filmed (times are given at the top in hours and minutes) and identified by time-lapse microscopy. Cells were stained with anti-GFP (centrin::GFP) and anti–C-Nap1 antibodies. Freestanding mother centrioles (or singlets) exhibit a 1:1 ratio of centrin and C-Nap1 foci, whereas duplicated centriole pairs (or doublets) display a 2:1 ratio. Our knockdown experiments blocked centriole duplication in >70% of cells that had gone through mitosis during the recording period. In these cells, either one or two mother centriole singlets were left (singlets; see C), depending on when the RNAi had worked in each cell, either one or two cell cycles before. The remaining ∼25% of cells were unaffected and still had two pairs of engaged centrioles (doublets; not depicted; see C). Note that when the expression of hSas-6R was turned on (DOX+), most of centriole singlets were able to duplicate in S phase (BrdU) and became doublets. (C) Quantification of centriole configuration and duplication for these S/G2 cells. Error bars indicate standard deviations from three independent experiments. The minus sign indicates lack of doxycycline treatment (no hSAS6).