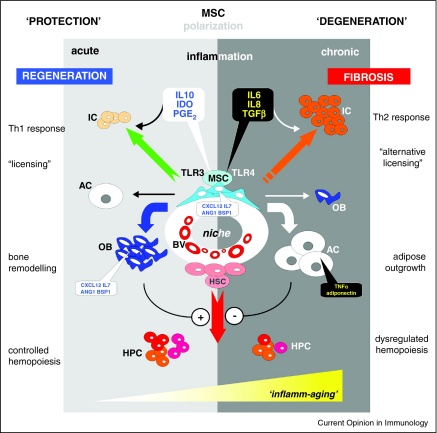
Figure 1.
-
(i)secrete indoleamine 2,3-dioxygenase (IDO), interleukin 6 (IL6), interleukin 8 (IL8), interleukin 10 (IL10), prostaglandin E-2 (PGE2), transforming growth factor beta (TGFβ) after activation of toll-like receptors 3 or 4 (TLR3, TLR4) to act on immune cells (IC) to trigger specific T helper (Th) cell responses;
-
(ii)to convey under low or transient inflammatory stimuli the differentiation of osteoblasts (OB) while at the same time checking progression of adipogenic cells (AC); contrastingly within a chronically inflamed periphery, the osteogenic fate of MSC progeny is stalled and adipogenic differentiation upholstered; the latter further propagate an inflammatory milieu through further excretion of tumor necrosis factor alpha (TNFα) or adiponectin;
-
(iii)to support the emergence and diversity of hematopoietic precursor cells (HPC) through strong osseous structures in a protected environment while stem cell proximities destined to degenerate by adipose outgrowth hinder the advent of HPC formation.
Deteriorating long-lasting chronic inflammation is frequently observed in biological communication systems at advanced age, for which the frequently used expression ‘inflamm-aging’ has been coined.