Figure 2.
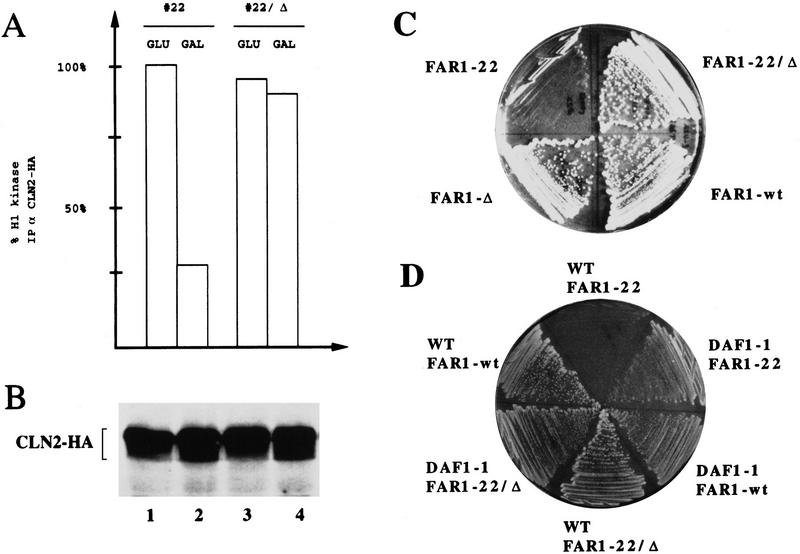
Cells producing Far1-22p arrest in G1 by inhibition of the G1 kinase, Cdc28p–Clnp. (A,B) Cln2p tagged at its carboxyl terminus with three copies of the HA epitope (Cln2–HA) was immunoprecipitated from extracts prepared from cells (YMT263) expressing either Far1-22p or for control, an inactive mutant form of Far1p, Far1-22/Δ285–350, from the inducible GAL promoter. Cells were grown in media containing galactose (GAL, GAL promoter on) or glucose (GLU, GAL promoter off). Cln2–HAp-associated kinase activity was measured with histone H1 as a substrate and quantified (A). The kinase activity associated with Cln2–HAp from cells grown in glucose was normalized to 100%. Similar amounts of Cln2–HAp were immunoprecipitated in each assay as shown by immunoblotting (B). (C) Growth inhibition caused by production of Far1-22p was dependent on the ability of Far1p to bind to the Cdc28p–Clnp kinase. The following Far1p proteins were analyzed: wild-type Far1p, Far1-22p, Far1-Δ285–350, which is unable to bind to Cdc28p–Clnp (Peter et al. 1993), and Far1-22/Δ285–350. (D) Growth inhibition caused by expression of Far1-22p was rescued by co-overexpression of a stable G1-cyclin, Cln3p (DAF1). DAF1-1 cells (IH2517), which express a stable form of the G1 cyclin Cln3p or isogenic wild-type cells (IH2518), were transformed with plasmids expressing either wild-type Far1p, Far1-22p, or Far1-22/Δ285–350p from the inducible GAL promoter.