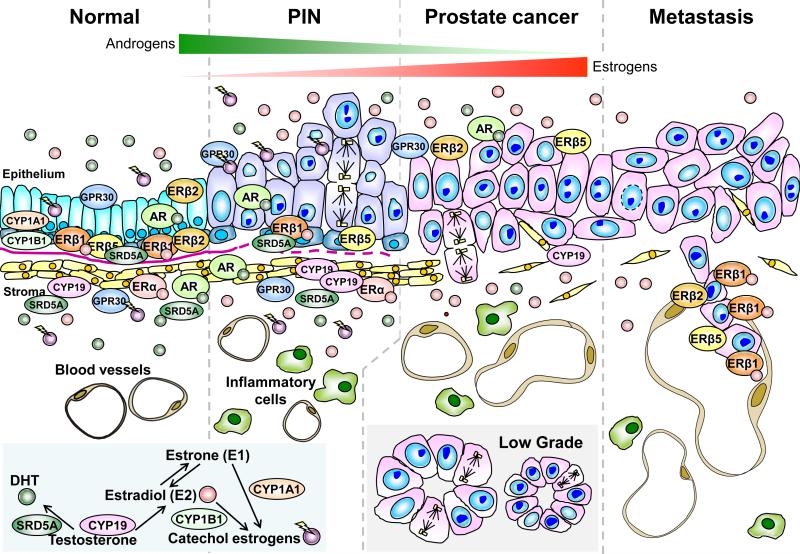
Figure. 1.
Expression of various hormone receptors and levels of hormones during the development and progression of prostate cancer (PCa). In the normal human prostate (far left panel), androgen receptor (AR), estrogen receptor alpha (ERα), different estrogen receptor beta (ERβ) isoforms, and G protein–coupled receptor 30 (GPR30) are expressed differentially in the stroma, and luminal and basal epithelium of the prostate. The epithelial cell compartment expresses only ERβ and AR, whereas the stromal compartment expresses both ERα and AR. GPR30 is expressed in both compartments. During tumor progression from high grade PIN (second to the far left panel) to prostate cancer (second to the far right panel), expression of AR and ERα remains unchanged, while expression of the antiproliferative ERβ1 is lost in the basal epithelial cells along with the disruption of the basement membrane (pink line). Two ERβ isoforms, ERβ2 and ERβ5, which have been shown to have pro-metastatic potential, are expressed in the normal epithelium, but their co-expression in PCa specimens is associated with a poor prognosis and shorter metastasis-free survival. In prostate metastases (far right panel), ERβ1 is re-expressed at high levels. Other non-canonical ERs such as GPR30 and ERRs are differentially expressed in normal and malignant prostate tissues but their roles in prostate carcinogenesis remains to be elucidated. Throughout the development and progression of PCa, tissue estrogen levels [estradiol-17β (E2) and estrone (E1)] increase with concomitant decreases in levels of 5α-dihydrotestosterone (DHT). These changes are due in part to an increase in CYP19 (aromatase) activity and a loss of SRD5A (5α-reductase) expression in the primary site (insert). SRD5A expression is completely lost in metastases. Additionally, an age-related reduction in testicular testosterone (T) synthesis, an increase in levels of sex hormone–binding globulin, and increases in adiposity and peripheral aromatase activity, collectively contribute to an increase in the estrogen to androgen ratio in circulation that further raise prostatic estrogen levels. In summary, a life-time over-exposure to estrogens and/or catecholestrogens could be an etiological factor in prostate carcinogenesis. The localizations of these enzymes as well as mediators of estrogen/androgen actions are indicated.