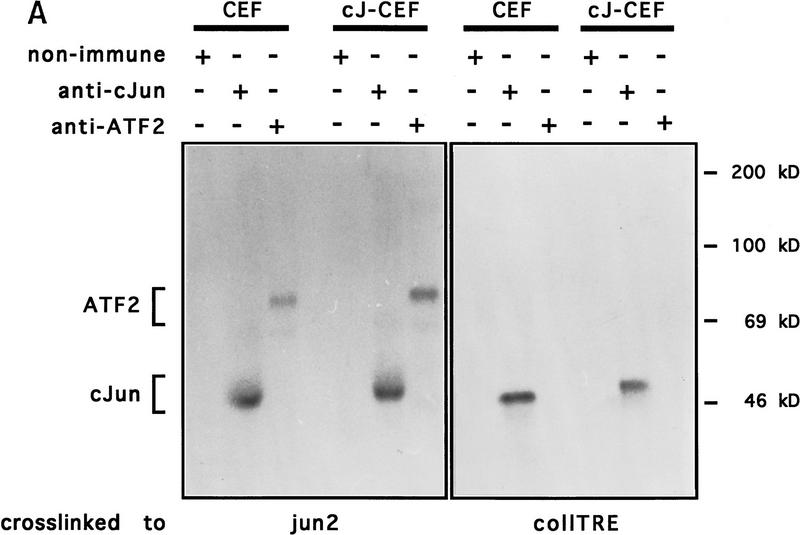
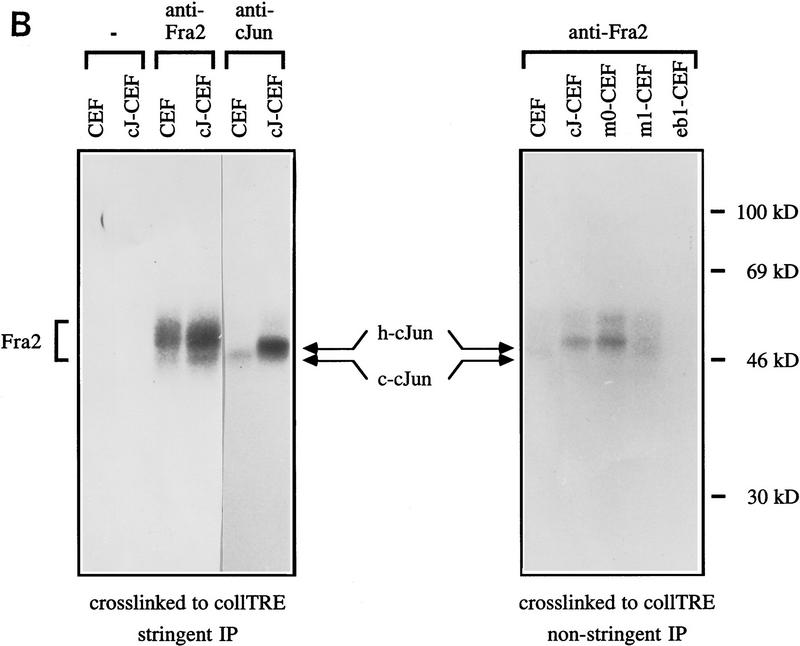
Figure 6.
c-Jun:ATF2 and c-Jun:Fra2 heterodimeric complexes in CEF cells. Immunoprecipitation of DNA-bound c-Jun, Fra2, and ATF2 from untransformed (CEF) and human c-Jun-transformed CEFs [wild-type c-Jun (cJ-CEF); c-Jun–m0 (m0-CEF); c-Jun–m1 (m1-CEF); c-Jun–eb1 chimera (Castellazzi et al. 1993, eb1-CEF)] extracts after covalent cross-linking to either the jun2 or coll–TRE oligonucleotides. CEF cell extracts were incubated with BrdU- and 32P-labeled DNA probes, cross-linked by UV-irradiation, and diluted in a mild (B) or stringent (A,B) immunoprecipitation buffer (see Materials and Methods). Immunocomplexes with antibodies as indicated were resolved on 12% SDS-PAGE and visualized by autoradiography. (A) The addition of antibody or nonimmune control antibody is shown above the gel plot. Note that endogenous chicken c-Jun migrates faster than human c-Jun. In cJ-CEF, the endogenous expression is repressed (see also Fig. 7). (B) To show the difference in c-Jun levels coprecipitating with Fra2 under nonstringent condition (right), relatively short exposures of the nonstringent precipitations are presented. Note that anti-Fra2 coprecipitates heterodimers of Fra2 with endogenous chicken c-Jun if the cross-links were done with CEF or m1-CEF extracts, heterodimers with human c-Jun only with cJ-CEF and m0-CEF extracts. No heterodimers with Fra2 are formed in the control extracts from Jun–eb1-CEF transformants as eb1–bZip mediates only homodimerization (Castellazzi et al. 1993). (−) Nonimmune serum.