Fig.5.
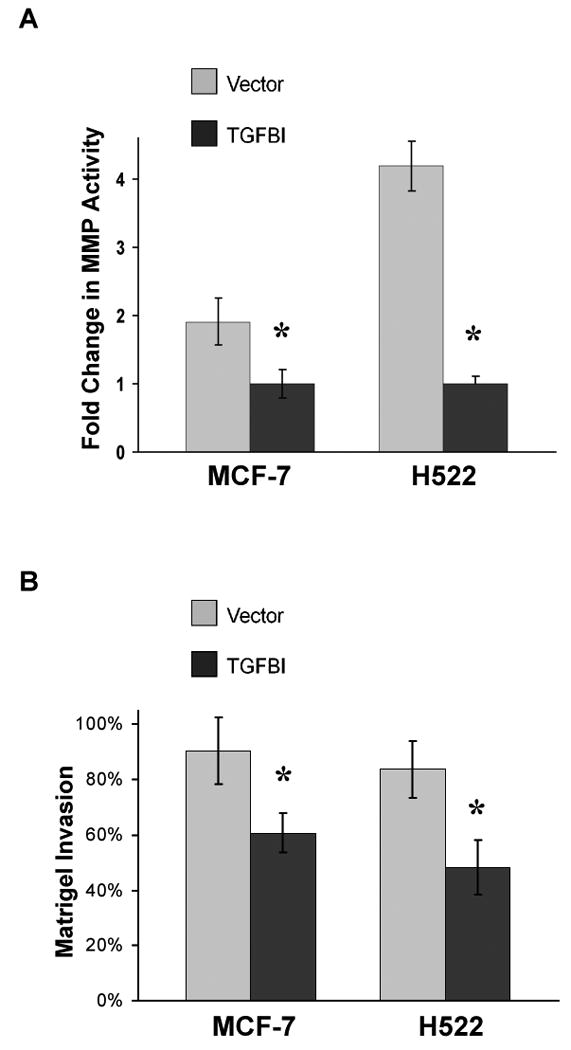
In vitro matrix metalloproteinase activity and invasion ability in cells expressing TGFBI. (A) Activity of MMP-2 and 9 in cell culture supernatant was measured using a MMP-2 and 9 gelatinase activity assay kit. The biotinylated gelatinase substrates were cleaved by active MMPs in the samples and the fragments were added to a biotin-binding plate. The digested but unbound fragments were removed by washing; whereas the bound undigested biotin-labeled gelatinase substrates were detected with streptavidin-enzyme complex producing a colored product measured at 450 nm. In vitro MMP-2 and 9 activities were significantly decreased in TGFBI-expressing cells. Data are mean±SD from three independent experiments and are presented as the fold change compared with vector control. *, p<0.05, compared to vector controls. (B) Invasion ability was measured using a matrigel invasion chamber. Cells in serum-free DMEM were seeded in an invasion chamber and serum-containing DMEM was added to the lower well. After incubation for 24 h, non-invading cells were removed from the upper surface and the cells at the bottom were fixed, stained with crystal violet and OD was read at 595 nm. In vitro invasion activities were significantly impaired with the expression of TGFBI in MCF-7 and H522 cells. Data are mean±SD from three independent experiments with values for cells invading through Matrigel insert membrane given as a percentage of the cell migration through control insert membranes. *, p<0.05, compared to vector controls.
