Figure 1.
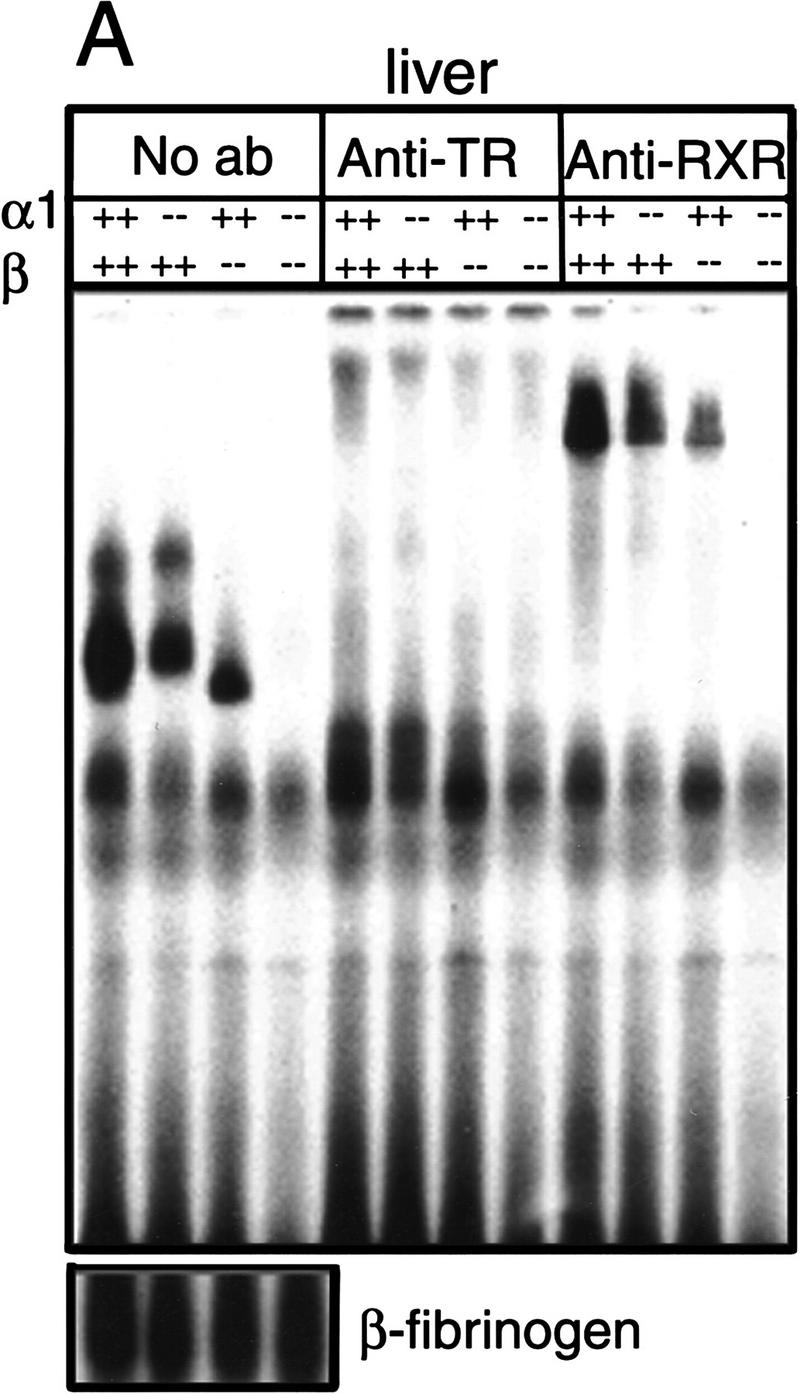
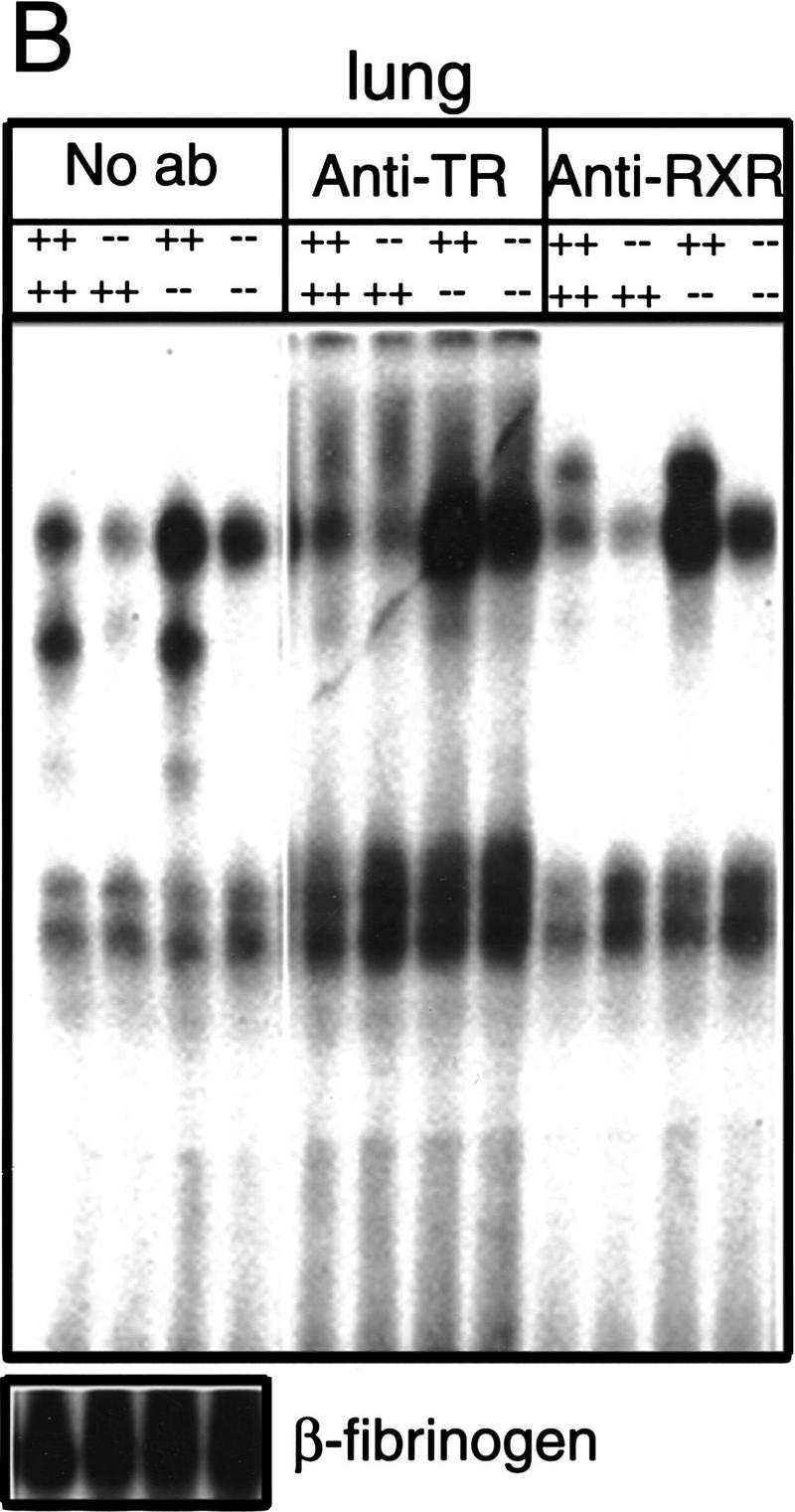
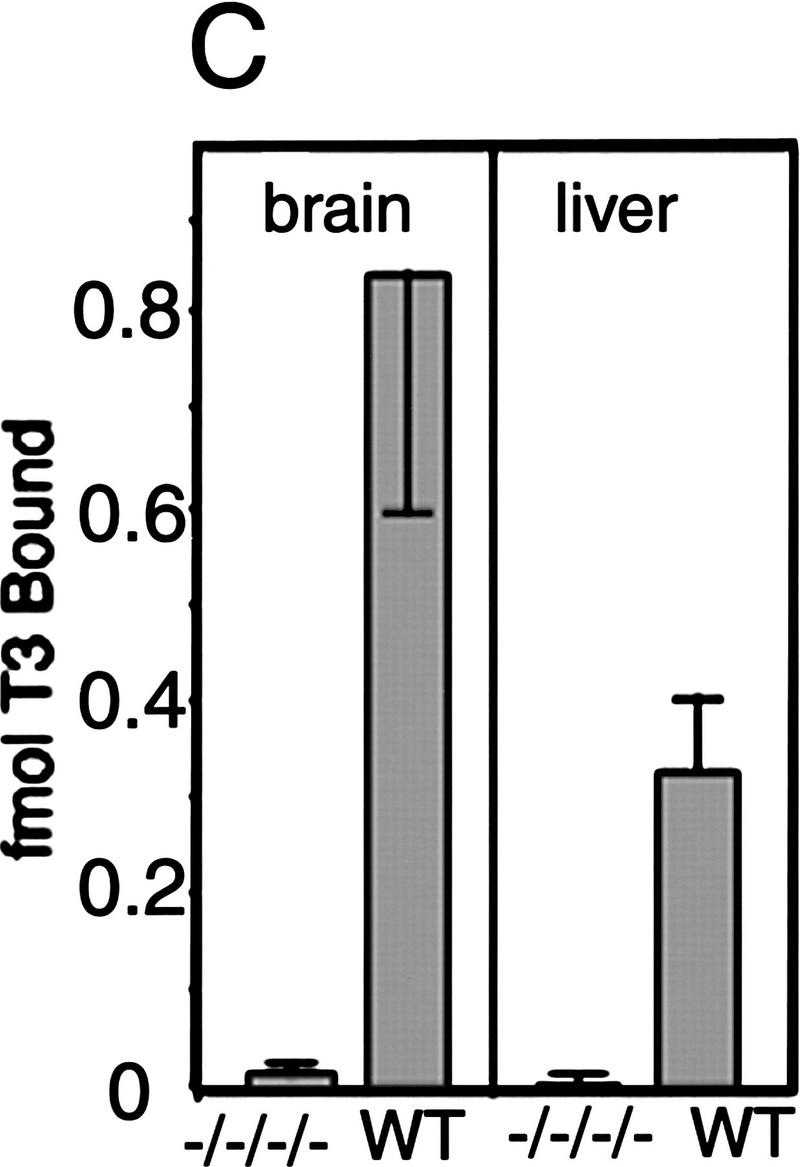
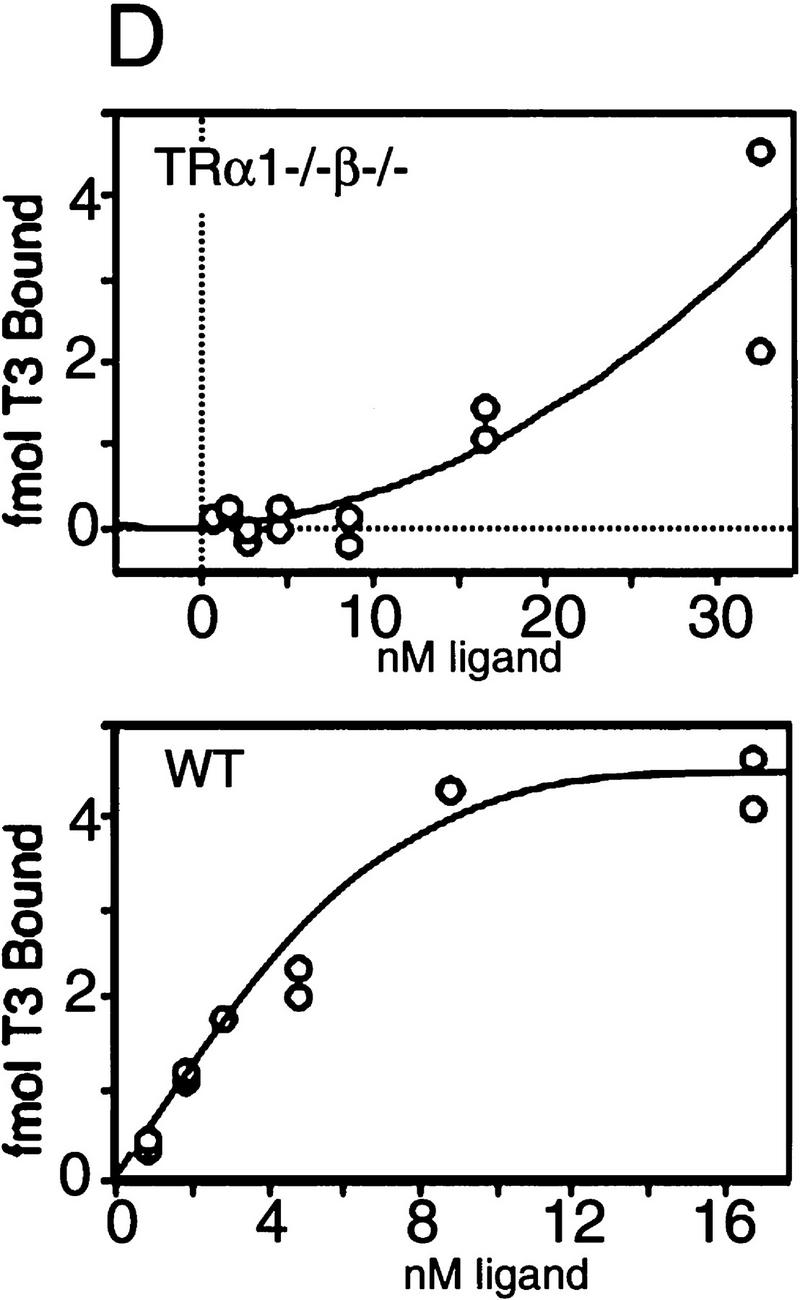
Absence of detectable T3 receptors in nuclear extracts from TRα1−/−β−/− mouse tissues. (A,B) Gel mobility shift experiments showing absence of TR proteins that can bind the F2 T3 response element in nuclear extracts from liver (A) and lung (B) of TRα1−/−β−/− mice. Specific TR-containing bands were identified by addition of antibodies against TR (anti-TR) or RXR (anti-RXR); (No ab) No antibodies added. Parallel analysis of samples with a β-fibrinogen probe demonstrated the integrity of other DNA-binding proteins in TR-deficient samples. (C) Absence of a specific T3 binding capacity in nuclear extracts from brain and liver of TRα1−/−β−/− mice. The 125I-labeled T3 binding capacity of TRα1−/−β−/− and wild-type samples is shown after subtraction of the binding that was not competed by a 1000-fold excess of cold T3 (Torresani and DeGroot 1975). (D) Absence of a saturable, specific T3 binding capacity in nuclear extracts from TRα1−/−β−/− mice. Nuclear extracts were prepared from mice that had been made hypothyroid to exclude the possibility that residual T3 binding sites were present but were masked by prior saturation by the excessive T3 levels in TRα1−/−β−/− mice (see Fig. 2A).
