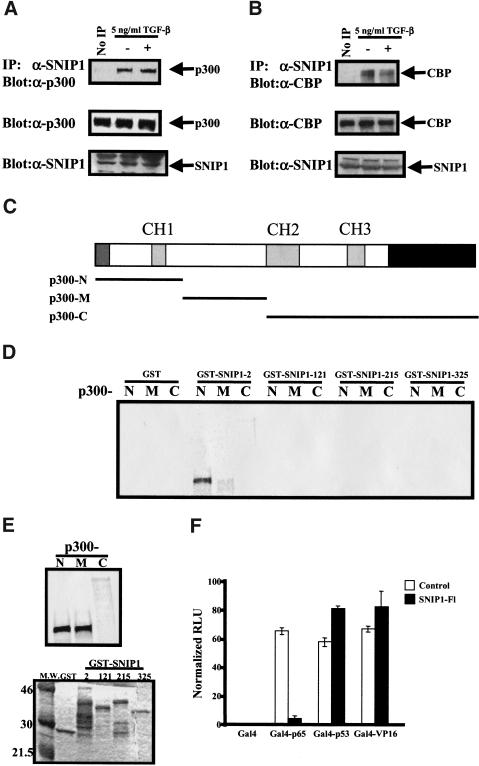
Figure 6.
Endogenous SNIP1 interacts with endogenous CBP/p300 through its amino terminus. NMuMg cell lysates treated with or without TGF-β were subjected to immunoprecipitation using α-SNIP1 and blotted with α-p300 (A) or α-CBP (B). The expression of these proteins in the cells was monitored by direct immunoblotting as shown at bottom. (C) Schematic diagram of p300 and location of the deletion constructs used to make an in vitro-transcribed/translated product. (D) p300 deletion constructs from the above diagram were used to make protein using reticulocyte lysate. The p300 deletion proteins were used in in vitro-binding assay using various bacterially expressed GST fusion of SNIP1 deletions constructs as indicated. After extensive washing, the beads were subjected to SDS-PAGE and autoradiography. (E) A total of 25% of the reticulocyte lysates used in the reaction was subjected to standard SDS-PAGE and autoradiography to detect the amounts used in each reactions (top). A total of 25% of GST fusion proteins used in the experiments was subjected to standard SDS-PAGE and stained as per experimental protocol to control for equal loading of GST proteins in the experiments (bottom). (F) NMuMg cells were cotransfected with pG5E1B with (+) or without (−) 0.5 μg of HA–SNIP1-FL, as indicated along with Gal4 fusion proteins of p65, p53, and VP16. Results are expressed as means (±s.d) of triplicate assays, normalized for transfection efficiency using β-Galactosidase activity.