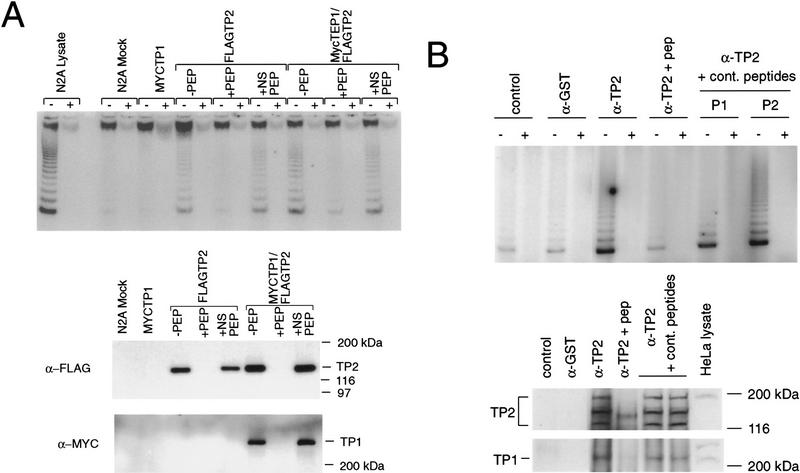
Figure 5.
TP2 interacts with TP1 in HeLa cells and transfected neuroblastoma cells. (A) Neuroblastoma cells were mock-transfected (N2A Mock) or transfected with MYC–TP1 alone, FLAG–TP2 alone, or MYC–TP1 and FLAG–TP2. Lysates plus (+) or minus (−) RNase were immunoprecipitated with anti-FLAG antibody, the precipitates were resolved by SDS-PAGE, and the Western blot was probed with anti-Myc and anti-FLAG antiserum. The peptide competitions on the FLAG immunoprecipitates were −PEP, no peptide added; +PEP, plus FLAG peptide; and +NS PEP, plus nonspecific peptide (Myc peptide). The mobility of the protein markers is shown at right. (B) Immunoprecipitations with TP2 (α-TP2) or control antiserum (rabbit α-mouse; control) and anti-GST (α-GST) were assayed for telomerase activity (top panel), Western blotting using anti-TP2 antiserum (middle), and anti-TP1 antiserum (bottom). Immunoprecipitation with anti-TP2 antiserum was also performed in the presence of TP2-specific peptide (α-TP2 + pep) and two different nonspecific peptides (to TP1; see Materials and Methods). In the bottom two panels, the samples are in the same order as above; the position of the protein markers are indicated at right, and the endogenous TP1 and TP2 proteins are indicated at left of each panel.