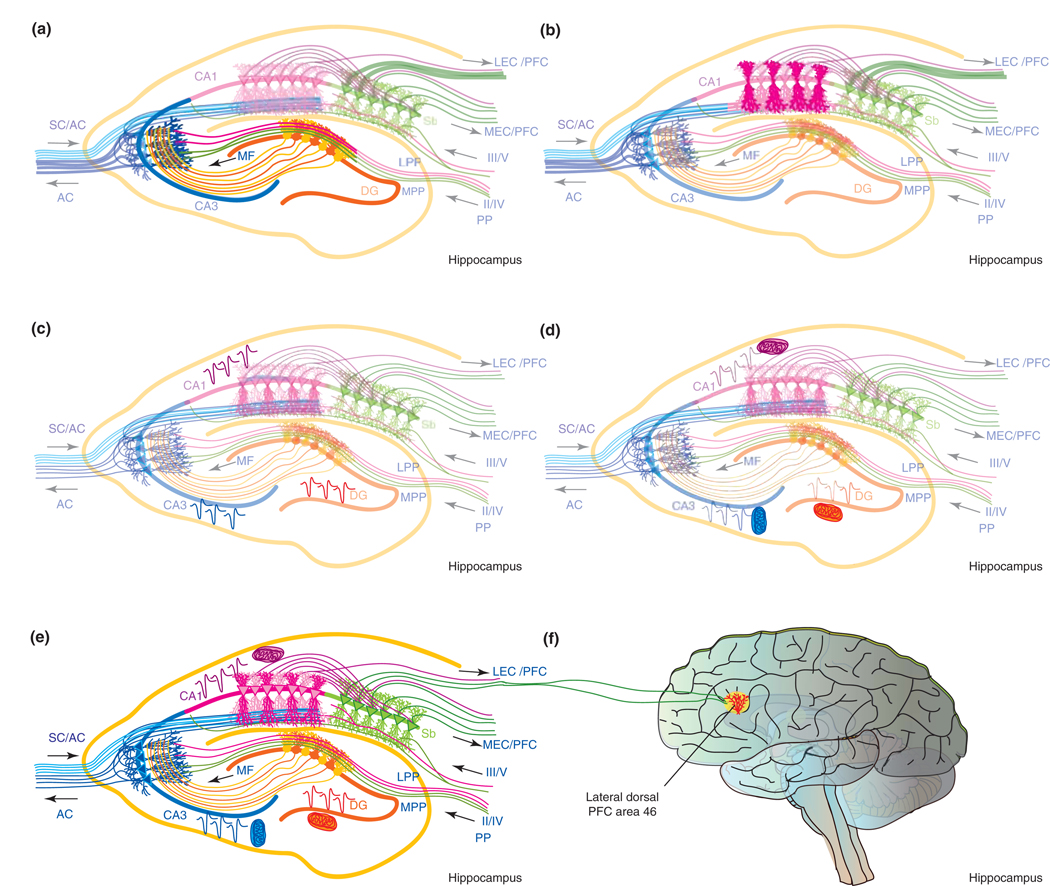
Figure 1.
Estrogen induces cellular, morphological and synaptic plasticity. (a) Estrogen (17β-estradiol; E2) enhances cellular plasticity via increased proliferation of neural progenitor cells within the subgranular zone, which are then integrated into the granule cell layer of the dentate gyrus (DG) (E2-induced newly generated cells are depicted as dark orange). Functionally new dentate granule cells contribute to the association of stimuli that are separated in time, a function that is a hippocampal-dependent type of associative learning and memory [69]. (b) Morphological plasticity is enhanced by E2 within the dentate and CA1 regions. E2 increased dendritic spines in the outer molecular layer of the dentate gyrus [6] (represented by the dark orange dendritic tree). Within the CA1 region, E2 increased spines of apical dendrites (dark pink dendritic tree) within the stratum radiatum, which receives excitatory input from CA3 neurons via the Schaffer collaterals [9] (blue pathway). The E2-induced increase in multiple synaptic boutons arising from CA3 Schaffer collateral axons synapsing onto CA1 apical dendrite spines [28] would predict an increase of either efficiency and/or capacity for information processing. Furthermore, the clustering of E2-inducible multisynaptic boutons [28] would indicate selective enhancement of input from particular CA3 cells. (c) E2 increased the field excitatory postsynaptic potential (fEPSP) within each subfield of the trisynaptic pathway (depicted in dark EPSPs in each subfield) and the dentate gyrus (represented by dark orange dendritic tree and fEPSP, respectively). E2 enhanced mossy-fiber input to CA3 while more robustly potentiating the AC fiber system of CA3 [34] (shown in blue). Potentiating both mossy-fiber and AC systems would be predicted to enhance CA3 associative memory to enhance the retrieval function of CA3 such that fewer elements (i.e. a partial representation) of a memory would be required for the whole memory to be retrieved [34]. In the CA1 subfield E2 potentiated both fEPSP and long-term potentiation [34], which would predict increased output to the subiculum (depicted in green). (d) E2 significantly increases aerobic glycolysis and mitochondrial function in the brain (depicted by dark mitochondria associated with fEPSP) [46,70,71]. The increase in glucose metabolism and mitochondrial function provide the energetic fuel and increased ATP to sustain increases in cellular, morphological and synaptic plasticity. (e) Collectively, E2 promotes cellular, morphological and synaptic plasticity in the hippocampus while simultaneously increasing the ATP necessary for enhanced plasticity. E2-induced increases in hippocampal plasticity impacts the output from subiculum to multiple brain regions including the PFC. (f) The lateral subiculum can project to area 46 of the lateral dorsal PFC [72], thereby providing a neuroanatomical connection between the information processing of the hippocampus and the temporal organization of behavior and reasoning governed by PFC. E2 significantly increased spine density of basal and apical dendrites of PFC neurons [6]. Estrogen-inducible increases in hippocampal and PFC plasticity predict that estrogen would preferentially affect cognitive tasks of greater complexity, temporal demand and associative challenge. Abbreviations: LEC, lateral entorhinal cortex; LPP, lateral perforant path; MEC, medial entorhinal cortex; MF, mossy fiber; MPP, medial perforant path; PP, perforant path; Sb, subiculum; SC, Schaffer collaterals. III/V and II/IV refer to the entorhinal cortex layers that feed into the pathways projecting to the hippocampus. Hippocampal scheme modified from www.bristol.ac.uk/synaptic/info/pathway/hippocampal.htm.