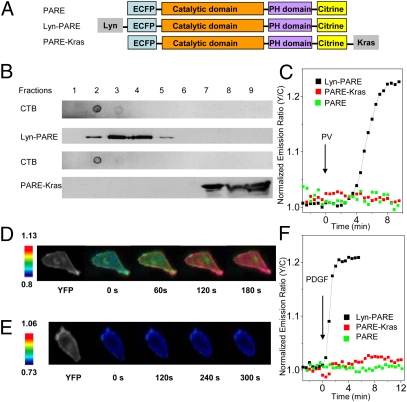
Fig. 1.
Development and characterization of PDK1 activation reporters. (A) The PDK1 activation reporter PARE was generated with full-length PDK1 sandwiched by a pair of fluorescent proteins, ECFP and citrine. PARE was targeted to membrane rafts by using a targeting motif derived from Lyn kinase and to nonraft regions of the plasma membrane by using a targeting motif derived from Kras. (B) The localization of Lyn-PARE and PARE-Kras was confirmed by sucrose density gradient fractionation. Total cell lysates from HEK 293 cells overexpressing Lyn-PARE or PARE-Kras were subjected to sucrose density gradient fractionation, followed by Western blotting. Cholera toxin subunit B (CTB) was used as a raft marker. The reporters were detected with an anti-GFP antibody. (C) Representative time courses showing the responses of PARE (n = 5), Lyn-PARE (n = 5), and PARE-Kras (n = 4) in serum-starved NIH 3T3 cells after stimulation with 100 μM PV. (D) Pseudocolor images showing the response of Lyn-PARE to 50 ng/mL PDGF in NIH 3T3 cells. The yellow fluorescence image (Left) shows the localization of Lyn-PARE. (E) Pseudocolor images showing the null response of PARE-Kras to 50 ng/mL PDGF in NIH 3T3 cells. The yellow fluorescence image (Left) shows the localization of PARE-Kras. (F) Stimulation of serum-starved NIH 3T3 cells with 50 ng/mL PDGF induced an emission ratio change in Lyn-PARE (n = 7), but not PARE (n = 5) or PARE-Kras (n = 6).