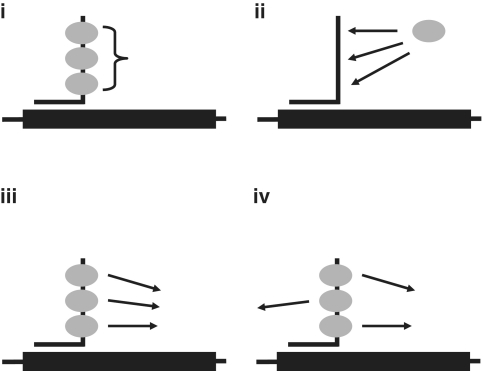
Figure 10.
Possible models for the effects of the length of the tail on the efficiency of splicing rescue. The diagram shows the bifunctional oligonucleotide and protein SRSF1, which has two RRM domains (ovals) and a RS-rich C-terminal region (shown unstructured). (i), a threshold number of molecules of protein are required to bind before activity is possible, possibly because proteins interact and binding is cooperative; (ii), the greater number of repeated motifs in the longer tails increases an otherwise extremely low affinity or probability of binding, (iii), the probability that a bound protein makes productive contacts is low, but occupancy by multiple molecules increases this probability, and (iv), bound proteins can make a number of different contacts and the presence of multiple proteins allows a number of concurrent interactions with other splicing components.