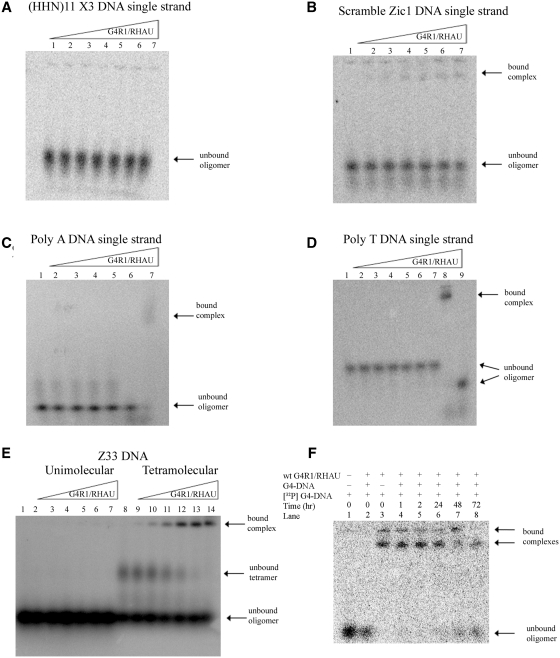
Figure 3.
Equilibrium binding GMSAs of purified recombinant G4R1/RHAU incubated with oligonucleotides not containing unimolecular G4-DNA shows that G4R1/RHAU does not tightly bind these substrates and suggests the enzyme has specificity for G4-DNA structures. (A–D) Phosphorimages of representative (of three repetitions) nondenaturing gel electropherograms of GMSAs; the top triangles indicate the direction of increasing G4R1/RHAU concentration. GMSA of (A) 1 pM 5′-[32P]-labeled d(pHHN)11 randomized DNA oligonucleotide incubated with increasing amounts of G4R1/RHAU (lane 1, 0 pM; lane 2, 30 pM; lane 3, 50 pM; lane 4, 75 pM; lane 5, 100 pM; lane 6, 150 pM; lane 7, 300 pM). (B) 1 pM 5′-[32P]-labeled Scrambled Zic1 single-stranded DNA oligonucleotide incubated with increasing amounts of G4R1/RHAU (lane 1, 0 pM; lane 2, 30 pM; lane 3, 50 pM; lane 4, 75 pM; lane 5, 100 pM; lane 6, 150 pM; lane 7, 300 pM). (C) 1 pM 5′-[32P]-labeled Poly A single-stranded DNA oligonucleotide incubated with increasing amounts of G4R1/RHAU (lanes 1–5, 0–300 pM; lane 6, Poly A DNA Zic1 47-mer G4-DNA incubated in the absence of G4R1/RHAU; lane 7, Poly A Zic1 DNA 47-mer G4-DNA incubated with 50 pM G4R1/RHAU). (D) 1 pM 5′-[32P]-labeled Poly T single-stranded DNA oligonucleotide incubated with increasing amounts of G4R1/RHAU (lanes 1–7, 0–300 pM; lane 8, Poly A Zic1 DNA 47-mer G4-DNA incubated with 30 pM G4R1/RHAU; lane 9, Poly A DNA Zic1 47-mer G4-DNA incubated in the absence of G4R1/RHAU). (E) 10 pM 5′-[32P]-labeled unstructured single-stranded Z33 oligonucleotide (lanes 1–7) or a mixture of unstructured and tetramolecular G4-DNA-structured Z33 (lanes 8–14) incubated with increasing amounts of G4R1/RHAU (lanes 1 and 8, 0 pM; lanes 2 and 9, 30 pM; lanes 3 and 10, 50 pM; lanes 4 and 11, 75 pM; lanes 5 and 12, 100 pM; lanes 6 and 13, 150 pM; lanes 7 and 14, 300 pM). (F) koff determination for a unimolecular G4-DNA bound to G4R1/RHAU. 1 pM 5′-[32P]-labeled Poly A Zic1 47-mer G4-DNA was bound to 200 pM G4R1/RHAU. Lane 1, 1 pM 5′-[32P]-labeled Poly A Zic1 47-mer G4-DNA in the absence of G4R1/RHAU; lane 2, 20 nM unlabeled Poly A Zic1 47-mer G4-DNA was added to G4R1/RHAU prior to the addition of 1 pM 5′-[32P]-labeled Poly A Zic1 47-mer G4-DNA; lane 3, 1 pM 5′-[32P]-labeled Poly A Zic1 47-mer G4-DNA was added to G4R1/RHAU in the absence of unlabeled Poly A Zic1 47-mer G4-DNA; lanes 4–8, 1 pM 5′-[32P]-labeled Poly A Zic1 47-mer G4-DNA was first added to G4R1/RHAU, then at t = 0, 20 nM unlabeled Poly A Zic1 47-mer G4-DNA was added and aliquots removed for GMSA at the times indicated. Half-life of binding was calculated to be 67 ± 9 h.