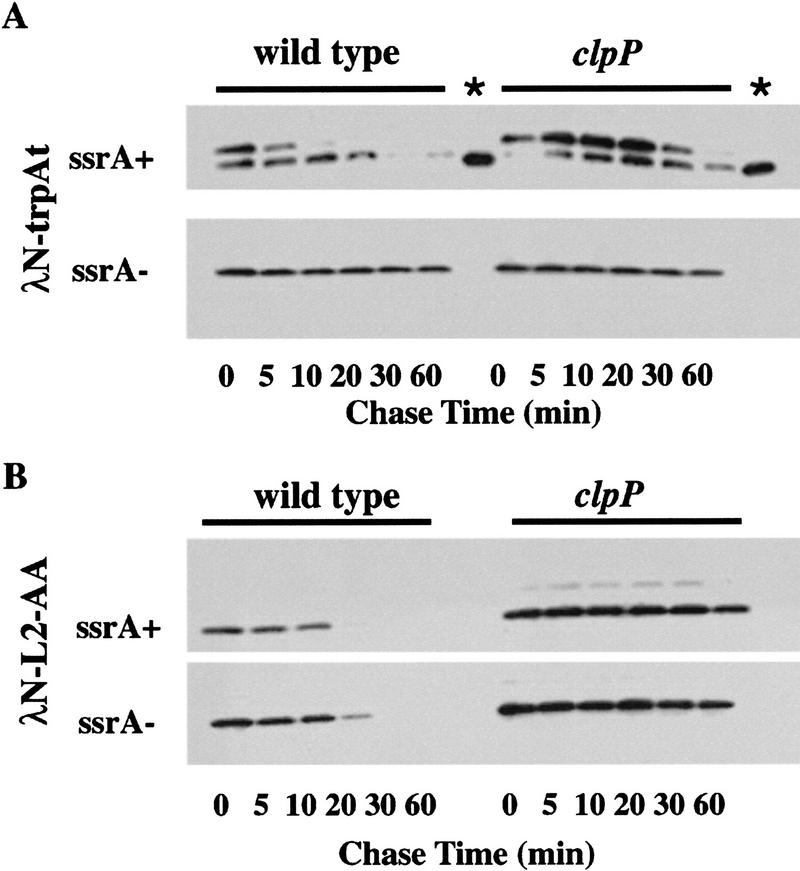
Figure 5.
Degradation of λN-trpAt and λN-L2-AA in vivo. (A) Western blots of λN–trpAt turnover following spectinomycin treatment in ssrA+ clp+ (SG22163), ssrA+ clpP::kan (SG22175), ssrA::cat clp+ (SG22183), and ssrA::cat clpP::kan (SG22184) strains. Note the presence of tagged and untagged variants of the λN-trpAt protein in ssrA+ strains. Lanes marked with an asterisk are untagged samples from the ssrA− host run on the same gel as ssrA+ samples to allow comparison of electrophoretic mobilities. Experimental procedures were the same as described in Fig. 3; 10%–20% gradient tricine gels (Novex) were used for electrophoresis. (B) Western blots of λN-L2-AA turnover. Strains and conditions were identical to those shown in A. In control experiments, λN-L2-DD expressed in the wild-type strain (SG22163) showed no significant turnover over 1 hr (data not shown). (C) Time courses of degradation of SsrA-tagged λN–trpAt protein (upper band in A) and λN-L2-AA in ssrA+ clp+ (SG22163) and ssrA+ clpP::kan (SG22175) strains. These data were obtained by scanning of the gels shown in A and B.