Figure 5.
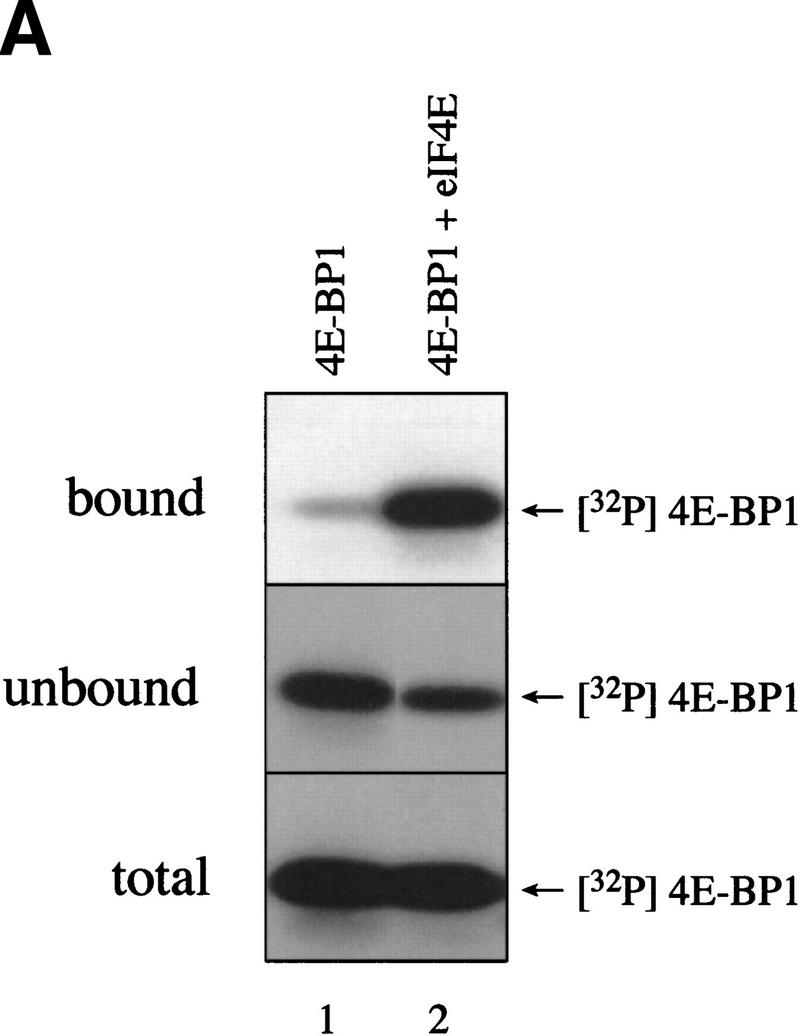
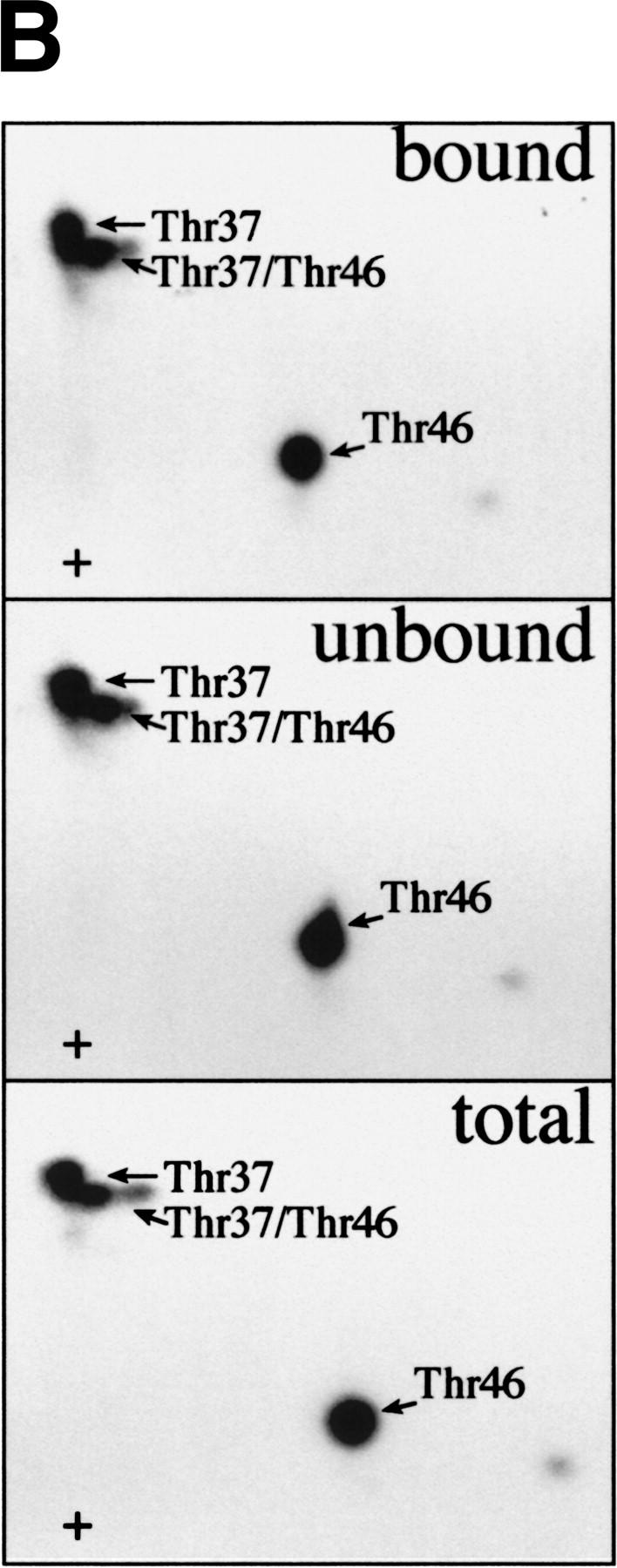
FRAP/mTOR phosphorylation of Thr-37 and Thr-46 does not disrupt the 4E-BP1/eIF4E complex. (A) 4E-BP1 alone, or a 4E-BP1/eIF4E complex, was incubated with FRAP/mTOR, as in Fig. 4. The kinase reaction was stopped and one-half of the material was immediately subjected to SDS-PAGE (bottom). The remaining material was incubated with m7GDP-agarose beads to purify eIF4E and associated proteins. The bound (top) and unbound (middle) fractions were analyzed by SDS-PAGE. (B) Phosphorylated 4E-BP1 (A, lane 2; bound, unbound, and total) was analyzed by two-dimensional phosphopeptide mapping.
