Abstract
The rapid synthesis of bicyclo[m.n.1]alkanone cores possessing quaternary carbon centers adjacent to a bridged ketone represents a significant synthetic challenge. This type of architectural feature is embedded in various complex biologically active compounds such as hyperforin and garsubellin A. Herein, we report a highly diastereoselective one-pot Diels–Alder reaction/Au(I)-catalyzed carbocyclization to generate bicyclo[3.3.1]alkanones in yields ranging from 48–93%.
Keywords: bicyclo[3.3.1]nonenone, carbocyclization, Diels–Alder, gold catalysis, one-pot process
Introduction
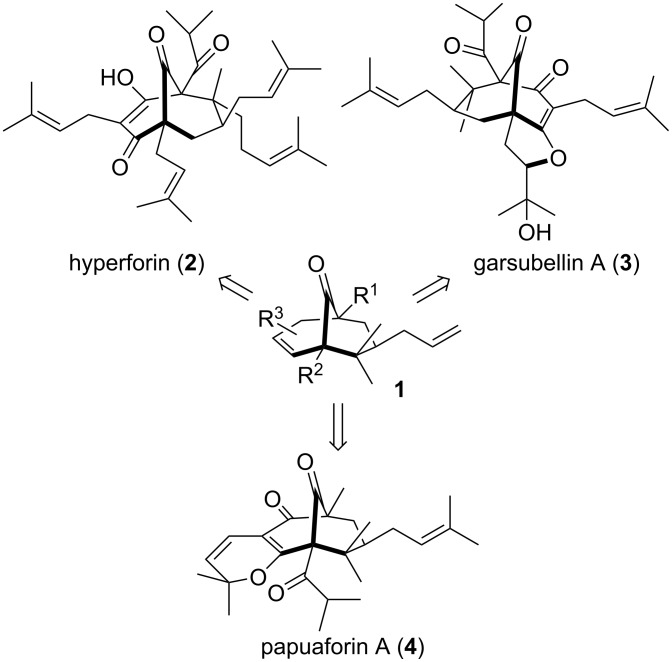
Highly oxygenated and densely substituted carbon-bridged medium sized rings such as 1 are commonly found in nature as structural frameworks of many important bioactive natural products, and in particular, polycyclic polyprenylated acetylphloroglucinols (PPAPs) (Figure 1) [1]. In the past decades, more than 100 PPAPs exhibiting a wide variety of biological activities (antibiotic, anti-HIV, anti-oxidant, etc.) have been isolated from Guttiferrea plants such as hyperforin (2) [2–6] and garsubellin A (3) [7–8]. The challenging synthesis of PPAP structures combined with their promising therapeutic potential has drawn attention from several research groups [9–12].
Figure 1.
Structures of naturally occurring PPAPs.
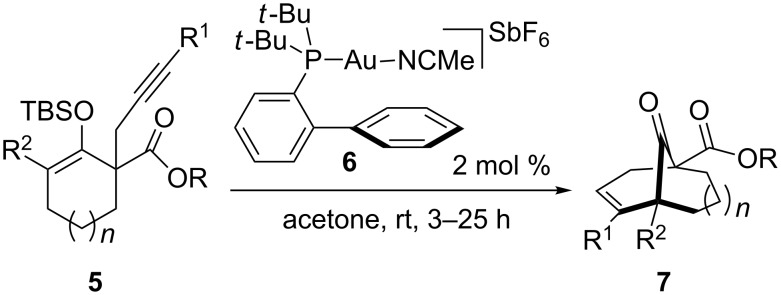
In 2009, we reported a mild and highly efficient method to generate carbon-bridged frameworks of various sizes through a gold(I)-catalyzed carbocyclization [13]. Although the cyclization of enol ether 5 can produce 5-exo and 6-endo products, we found that gold complexes 6, having bulky phosphine ligands such as 2-bis(tert-butylphosphino)biphenyl, gave exclusively the 6-endo-dig cyclized products 7 (Scheme 1). In the course of our studies directed towards the synthesis of naturally occurring PPAPs and related carbon-bridged ketone scaffolds, we envisioned that PPAP framework 1 could be generated via a Au(I)-catalyzed cyclization [14–22].
Scheme 1.
Gold(I)-catalyzed 6-endo-dig cyclization.
Results and Discussion
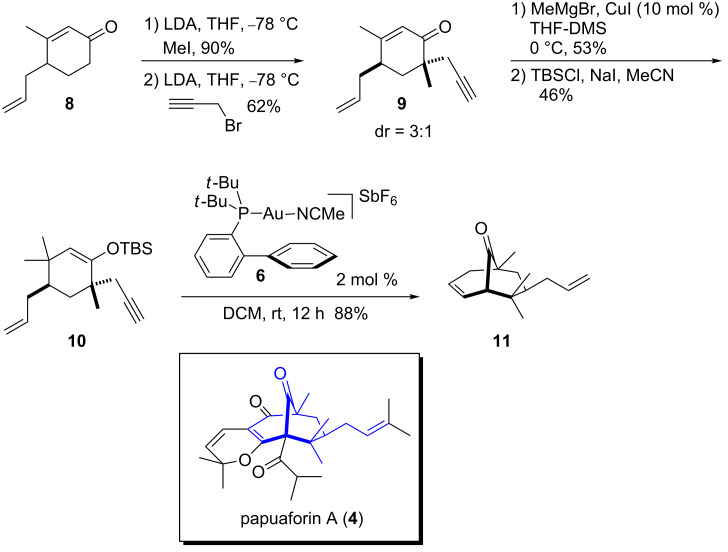
The synthesis began by a C-alkylation of enone 8 [23] using LDA and MeI to give the corresponding ketone in 90% yield (Scheme 2). A second alkylation to add the propargyl chain was carried out using LDA and propargyl bromide to afford 9 in 62% yield as an inseparable mixture of diastereomers (dr = 3:1). Subsequently, conjugate addition of methylmagnesium bromide in the presence of a catalytic amount of CuI provided the corresponding ketone in 53% yield. The ketone was then treated with TBSCl, NaI and triethylamine to give the desired silylenol ether 10 in 46% yield, which upon exposure to the Au(I) complex 6 (2 mol %) provided the desired bicyclo[3.3.1]nonenone 11 in 88% yield. It is important to note that the Au(I)-catalyzed cyclization proceeds in high yields in a sterically congested environment. The synthesis of the core of papuaforin (11) was achieved in five steps from enone 8.
Scheme 2.
Synthesis of papuaforin A core 4.
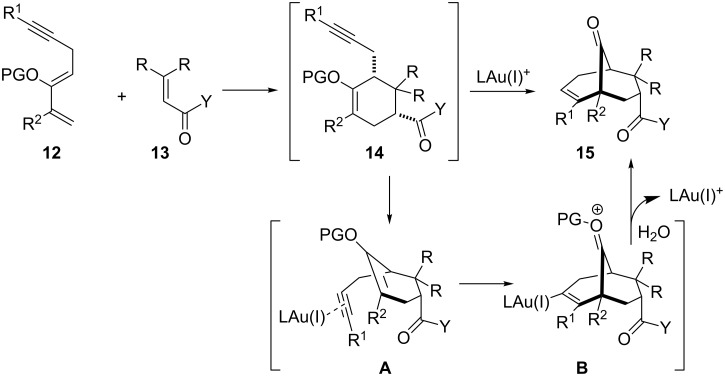
However, one might recognize that the low chemical yields encountered in some steps undermine the efficacy of the Au(I)-catalyzed cyclization approach. In order to solve this issue, we assumed that bicyclo[3.3.1]nonenone scaffolds can be directly obtained through an intermolecular Diels–Alder reaction/Au(I)-catalyzed 6-endo-dig carbocyclization (Scheme 3). Cycloaddition between diene 12 and dienophile 13 should provide the endo cycloadduct 14, which, in the presence of a gold(I) catalyst, would form the gold complex A. This undergoes a carbocyclization of enol ether [24–31] to afford intermediate B, which after proto-deauration and hydrolysis affords the bridgehead ketone 15. The attractive feature of this process resides in the ability to generate four new stereogenic centers and three new C–C bonds in one single operation.
Scheme 3.
Proposed domino Diels–Alder reaction/gold(I)-catalyzed cyclization.
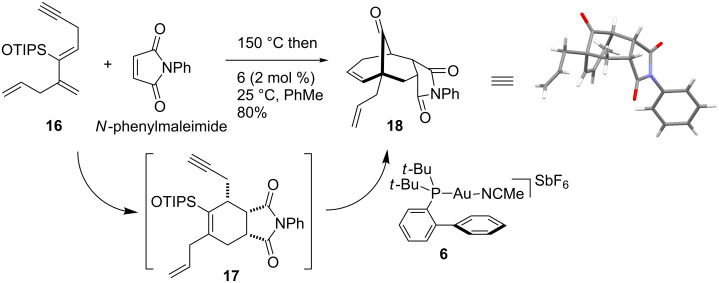
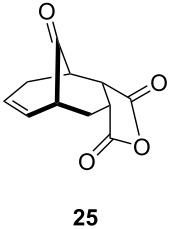
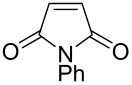
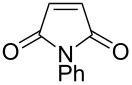
To validate the above hypothesis, diene 16 (Z-isomer) was heated with N-phenylmaleimide in toluene at 150 °C, for two hours, by microwave irradiation (Scheme 4) (see Supporting Information File 1 for experimental procedures). The solution containing the Diels–Alder adduct 17 was cooled down to room temperature and 2 mol % of Au(I) complex 6 was added. The bridgedhead ketone 18 was obtained in 80% yield as a single diastereomer. The relative stereochemistry of 18 was unambiguously established by X-ray analysis (see Supporting Information File 2). With this result in hand, we explored the scope of this sequential reaction (Table 1).
Scheme 4.
One-pot Diels–Alder cycloaddition/gold(I) catalyzed carbocyclization.
Table 1.
Results of the one-pot Diels–Alder reaction/Au(I)-catalyzed cyclization.
| entry | diene | dienophile | product (yield)a |
| 1 | 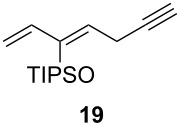 |
 |
 (93%) |
| 2 | 19 | 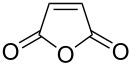 |
 (51%) |
| 3 | 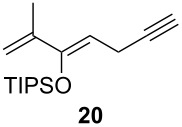 |
 |
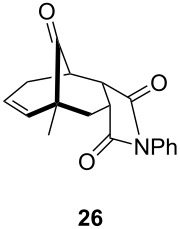 (88%) |
| 4 | 20 | 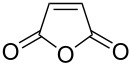 |
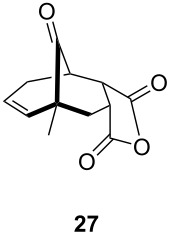 (50%) |
| 5 | 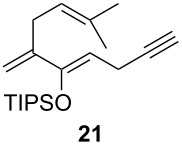 |
 |
 (81%) |
| 6 | 21 | 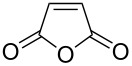 |
 (78%) |
| 7 | 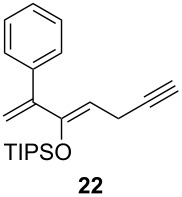 |
 |
 (77%) |
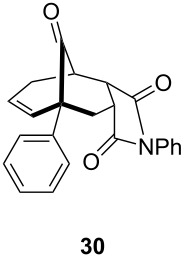
| 8 | 22 | 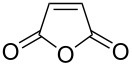 |
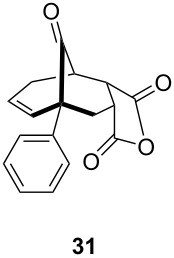 (48%) |
| 9 | 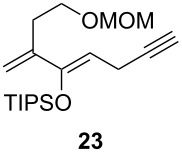 |
 |
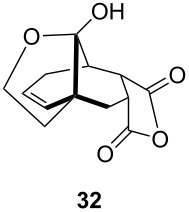 (56%) |
aIsolated yield and dr > 25:1 in all cases.
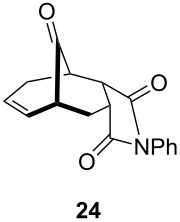
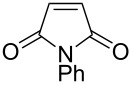
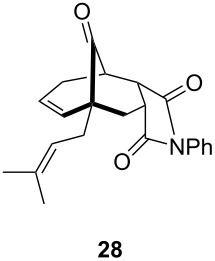
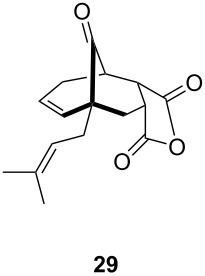
One-pot cycloaddition/cyclization of dienes 19 and 20 (Z/E = 6:1 ca.) with N-phenylmaleimide gave ketones 24 and 26 in 93 and 88% yield, respectively, as the sole diastereomers (Table 1, entries 1 and 3). The use of maleic anhydride as the dienophile also provided the desired products 25 and 27, albeit in lower yields of 51 and 50%, respectively (Table 1, entries 2 and 4). Prenylated diene 21 was smoothly converted to ketones 28 and 29 in 81 and 78% yield, respectively (Table 1, entries 5 and 6). Table 1, entries 7 and 8 reveal that the diene 22, bearing a phenyl group at C2, can be stereoselectively transformed into the desired bridgehead ketones 30 and 31 in 77 and 48% yields, respectively. Interestingly, hemiketal 32 was isolated in 56% yield, which suggests that the MOM group was cleaved during the Au(I)-catalyzed carbocyclization. It is important to note that the E-isomer of dienes 19–23 (minor compound) do not react with the dienophiles, but rather isomerized to the Z-form under the reaction conditions, thus, ensuring the formation of a single diastereomer.
To extend the scope of the reaction, other dienes possessing internal alkynes were also investigated (Table 2). It can be seen that large substituents at the alkyne terminal position did not affect the efficacy of the reaction. Intermolecular cycloaddition/Au(I)-catalyzed cyclization of aryl acetylene dienes 33–35 provided the desired ketones 37–39 in yields ranging from 68 to 91% (Table 2, entries 1–3). Remarkably, enyne 36 was converted to 40 in 79% yield (Table 2, entry 4).
Table 2.
One-pot Diels–Alder cycloaddition/Au(I)-catalyzed carbocyclization of internal alkynes.
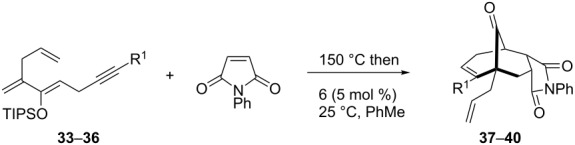 | |||
| entry | substituent R1 | product | yield (%)a |
| 1 | 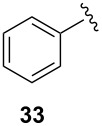 |
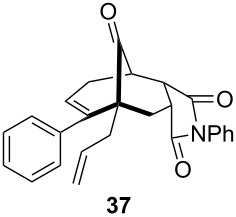 |
68 |
| 2 | 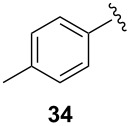 |
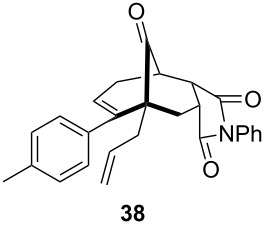 |
91 |
| 3 | 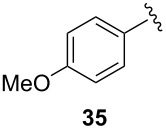 |
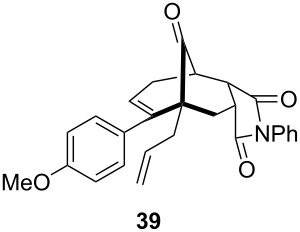 |
74 |
| 4 | 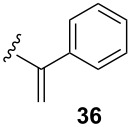 |
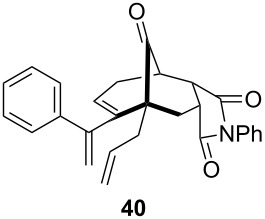 |
79 |
aIsolated yield and dr >25:1 in all cases.
Conclusion
In summary, we have developed an efficient stereoselective method for the construction of bicyclic[3.3.1]nonenone frameworks. This one-pot Diels–Alder/Au(I)-catalyzed carbocyclization process provides access to synthetically useful motifs that are found in numerous naturally occurring PPAPs. In addition, the Au(I)-catalyzed cyclization proved to be tolerant of a sterically crowded environment. Further studies to develop an enantioselective version of this reaction and its application to the total synthesis of hyperforin (2) and garsubellin A (3) are underway and will be reported in due course.
Supporting Information
Experimental procedures, characterization data, 1H NMR and 13C NMR spectra.
X-ray data of compound 18.
Acknowledgments
We thank the Natural Science and Engineering Research Council of Canada (NSERC), Merck Research Laboratories, Merck Frosst Canada, Boehringer Ingelheim (Laval), PREA, Canada Foundation for Innovation, Ontario Innovation Trust and the University of Ottawa for generous funding. F.B. thanks NSERC for post-graduate scholarship (CGS-D1).
This article is part of the Thematic Series "Gold catalysis for organic synthesis".
References
- 1.Ciochina R, Grossman R B. Chem Rev. 2006;106:3963. doi: 10.1021/cr0500582. [DOI] [PubMed] [Google Scholar]
- 2.Bystrov N S, Chernov B K, Dobrynin V N, Kolosov M N. Tetrahedron Lett. 1975;16:2791. doi: 10.1016/S0040-4039(00)75241-5. [DOI] [Google Scholar]
- 3.Müller W E, Singer A, Wonnemann M, Hafner U, Rolli M, Schäfer C. Pharmacopsychiatry. 1998;31:16. doi: 10.1055/s-2007-979341. [DOI] [PubMed] [Google Scholar]
- 4.Verotta L, Appendino G, Bombardelli E, Brun R. Bioorg Med Chem Lett. 2007;17:1544. doi: 10.1016/j.bmcl.2006.12.100. [DOI] [PubMed] [Google Scholar]
- 5.Gey C, Kyrylenko S, Henning L, Nguyen L-H D, Büttner A, Pham H D, Giannis A. Angew Chem, Int Ed. 2007;46:5219. doi: 10.1002/anie.200605207. [DOI] [PubMed] [Google Scholar]
- 6.Moore L B, Goodwin B, Jones S A, Wisely G B, Serabjit-Singh C J, Willson T M, Collins J L, Kliewer S A. Proc Natl Acad Sci U S A. 2000;97:7500. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuyama Y, Kuwayama A, Minami H. Chem Pharm Bull. 1997;45:947. doi: 10.1248/cpb.45.947. [DOI] [PubMed] [Google Scholar]
- 8.Fukuyama Y, Minami H, Kuwayama A. Phytochemistry. 1998;49:853. doi: 10.1016/S0031-9422(98)00126-5. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu Y, Shi S L, Usuda H, Kanai M, Shibasaki M. Angew Chem, Int Ed. 2010;49:1103. doi: 10.1002/anie.200906678. [DOI] [PubMed] [Google Scholar]
- 10.Kuramochi A, Usuda H, Yamatsugu K, Kanai M, Shibasaki M. J Am Chem Soc. 2005;127:14200. doi: 10.1021/ja055301t. [DOI] [PubMed] [Google Scholar]
- 11.Danishefsky S J, Siegel D R. J Am Chem Soc. 2006;128:1048. doi: 10.1021/ja057418n. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad N M, Rodeschini V, Simpkins N S, Ward S E, Blake A J. J Org Chem. 2007;72:4803. doi: 10.1021/jo070388h. [DOI] [PubMed] [Google Scholar]
- 13.Barriault L, Barabé F, Bétournay G, Bellavance G. Org Lett. 2009;11:4236. doi: 10.1021/ol901722q. [DOI] [PubMed] [Google Scholar]
- 14.Toste F D, Gorin D J. Nature. 2007;446:395. doi: 10.1038/nature05592. [DOI] [PubMed] [Google Scholar]
- 15.Jiménez-Núñez E, Echavarren A M. Chem Commun. 2007:333. doi: 10.1039/B612008C. [DOI] [PubMed] [Google Scholar]
- 16.Fürstner A, Davies P W. Angew Chem, Int Ed. 2007;46:3410. doi: 10.1002/anie.200604335. [DOI] [PubMed] [Google Scholar]
- 17.Hashmi A S K. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x. [DOI] [PubMed] [Google Scholar]
- 18.Gorin D J, Sherry B D, Toste F D. Chem Rev. 2008;108:3351. doi: 10.1021/cr068430g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z, Brouwer C, He C. Chem Rev. 2008;108:3239. doi: 10.1021/cr068434l. [DOI] [PubMed] [Google Scholar]
- 20.Arcadi A. Chem Rev. 2008;108:3266. doi: 10.1021/cr068435d. [DOI] [PubMed] [Google Scholar]
- 21.Skouta R, Li C-J. Tetrahedron. 2008;64:4917. doi: 10.1016/j.tet.2008.03.083. [DOI] [Google Scholar]
- 22.Shapiro N D, Toste F D. Synlett. 2010;5:675. doi: 10.1055/s-0029-1219369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson W S, McCarry B E, Markezich R L, Boots S G. J Am Chem Soc. 1980;102:352. doi: 10.1021/ja00521a057. [DOI] [Google Scholar]
- 24.Dankwardt J W. Tetrahedron Lett. 2001;42:5809. doi: 10.1016/S0040-4039(01)01146-7. [DOI] [Google Scholar]
- 25.Suhre M H, Reif M, Kirsch S F. Org Lett. 2005;7:3925. doi: 10.1021/ol0514101. [DOI] [PubMed] [Google Scholar]
- 26.Staben S T, Kennedy-Smith J J, Huang D, Corkey B K, LaLonde R L, Toste F D. Angew Chem, Int Ed. 2006;45:5991. doi: 10.1002/anie.200602035. [DOI] [PubMed] [Google Scholar]
- 27.Linghu X, Kennedy-Smith J J, Toste F D. Angew Chem, Int Ed. 2007;46:7671. doi: 10.1002/anie.200702695. [DOI] [PubMed] [Google Scholar]
- 28.Lee K, Lee P H. Adv Synth Catal. 2007;349:2092. doi: 10.1002/adsc.200700304. [DOI] [Google Scholar]
- 29.Minnihan E C, Colletti S L, Toste F D, Shen H C. J Org Chem. 2007;72:6287. doi: 10.1021/jo071014r. [DOI] [PubMed] [Google Scholar]
- 30.Kusama H, Karibe Y, Onizawa Y, Iwasawa N. Angew Chem, Int Ed. 2010;49:4269. doi: 10.1002/anie.201001061. [DOI] [PubMed] [Google Scholar]
- 31.Ito H, Ohmiya H, Sawamura M. Org Lett. 2010;12:4380. doi: 10.1021/ol101860j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental procedures, characterization data, 1H NMR and 13C NMR spectra.
X-ray data of compound 18.