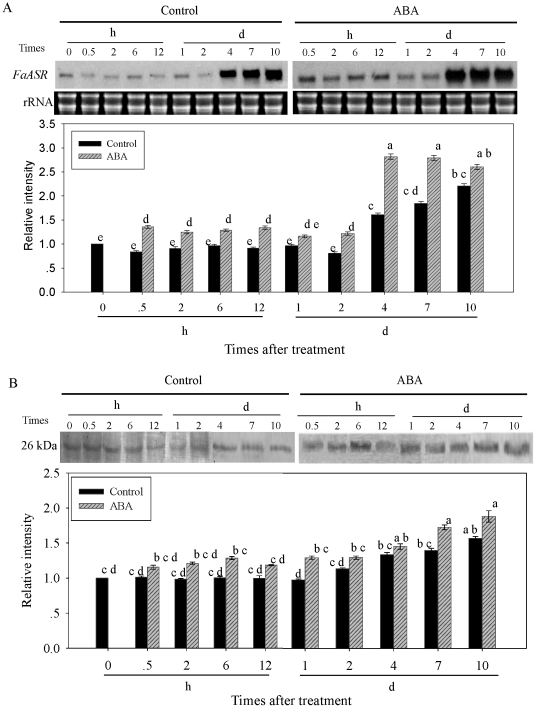
Figure 8. Changes in FaASR mRNA (A) and protein (B) accumulations after ABA treatment at the LG stage.
Selected fruits at the LG stage (about 15 days after post-anthesis) were dipped for 1 min in a solution containing 0 (control) or 100 µM ABA, and then sampled at 0, 0.5, 2, 6 and 12 h, 1, 2, 4, 7 and 10 days. In (A), total RNA (10 μg per lane) was used for northern blot analysis and hybridized with DIG-labeled probe, and ethidium bromide-stained rRNA was shown as the loading control. In (B), equal amounts of protein (30 μg per lane) were subjected to SDS-PAGE and then transferred to a nitrocellulose membrane. Thereafter, the FaASR protein amount was immunodetected by western blot using the anti-FaASR specific polyclonal antibody. The quantification of the northern or western blot bands was expressed in relation to the amount in control fruit sampled at time 0, which was set to 1. Vertical bars represented standard deviations (SD) of means (n = 3). Different letters indicated a statistical difference at the 5% level among data groups according to the Duncan's multiple range test.