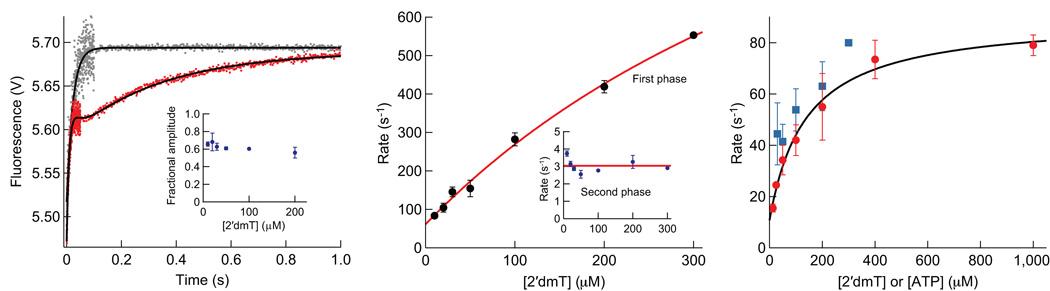
Figure 5.
Binding of 2′dmT to Kin6AA. (a) A complex of Kin6AA and microtubules was pre-formed and mixed in a stopped-flow apparatus with 2′dmT (Supplementary Methods). The resulting fluorescence signal (red) consisted of three sequential phases: a first phase of increasing fluorescence, a lag phase, and a second phase of increasing fluorescence. Fitting this signal required three exponential terms (black curve). Two terms, corresponding to the phases of increasing fluorescence, were associated with rate constants of 81.5 ± 21.0 s−1 and 3.0 ± 0.1 s−1. The third term was associated with a low-amplitude, decreasing phase, consistent with a lag, and a rate constant of 55.6 ± 24.8 s−1. The same experiment in the absence of microtubules (grey) produced a fluorescence increase with a single exponential phase with a rate constant of 45.0 ± 1.0 s−1. Inset: Fractional amplitude of the first phase vs. [2′dmT]. (b) Rate constant for the first phase of fluorescence increase vs. [2′dmT]. Data (black dots; mean ± s.e.m.) were fit to a hyperbola (red curve) that extrapolates to 61 ± 12 s−1 at zero [2′dmT] and is associated with a second-order rate constant of 2.6 ± 0.3 µM−1 s−1. Inset: Rate constant for the second phase of fluorescence vs. [2′dmT], which averages 3.0 ± 0.4 s−1 (red line) (c) Rate of initial decay of TMR fluorescence as a function of [ATP], compared to the rate of the lag phase in (a). Data (mean ± s.e.m.; N = 18–35; red dots) were fit to a rectangular hyperbola (black curve); the asymptotic rate at saturating ATP was 90 ± 4 s−1. The y-intercept of the fit at 11 ± 5 s−1 is interpreted as the rate at which a head rebinds to the microtubule. Rate constant for the lag phase vs. [2′dmT] (mean ± s.e.m.; N = 10–20; blue squares).