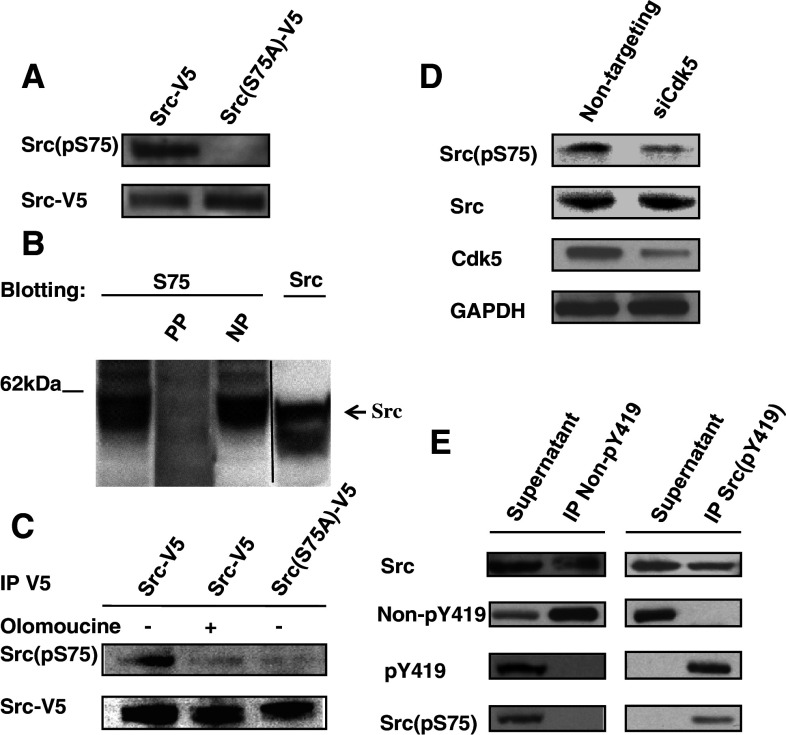
Fig. 5.
Cdk5-dependent phosphorylation of Src(S75) occurs in cells. a Human lens epithelial cells were transfected with Src-V5 or Src(S75A)-V5. Cell lysates were immunoprecipitated with V5 antibody and immunoblotted with phospho-specific antibody to Src(pS75). The phospho-specific antibody detected Src(pS75) in cells transfected with Src-V5, but not in those transfected with Src(S75A)-V5. b Cos7 cells were transfected with Src-V5 or Src(S75A)-V5, and immunoblotted to detect endogenous Src(pS75) in the presence or absence of the phosphorylated antigenic peptide (PP) or its non-phosphorylated counterpart (NP). The phosphorylated peptide, but not the non-phosphorylated peptide, completely blocked immunoreactivity. c Cos7 cells were transfected with Src-V5 or Src(S75A)-V5 and incubated for a total of 24 h with or without 15 μM olomoucine during the final 16 h. Cell lysates were immunoprecipitated with V5 antibody then immunoblotted to detect Src(pS75) and V5. The antibody detected significant Src(pS75) in Src-V5 transfected cells, but only trace amounts in olomoucine treated Src-V5 cells and Src(S75A)-V5 cells. d Lens epithelial cells were transfected with siRNA to suppress Cdk5 (siCdk5) or with non-targeting siRNA oligonucleotides (non-targeting). Cell lysates were immunoblotted with antibodies against Src(pS75), Src, Cdk5, and GAPDH. Src(pS75) expression was inhibited in siCdk5-transfected cells. e Cell lysates were immunoprecipitated with antibody specific for either inactive Src (Non-pY419) or active Src (pY419). The supernatant fractions and immunoprecipitated fractions were immunoblotted with antibodies against Src, Non-pY419, Src(pY419), and Src(pS75). This experiment is representative of three experiments with similar results