Figure 2.
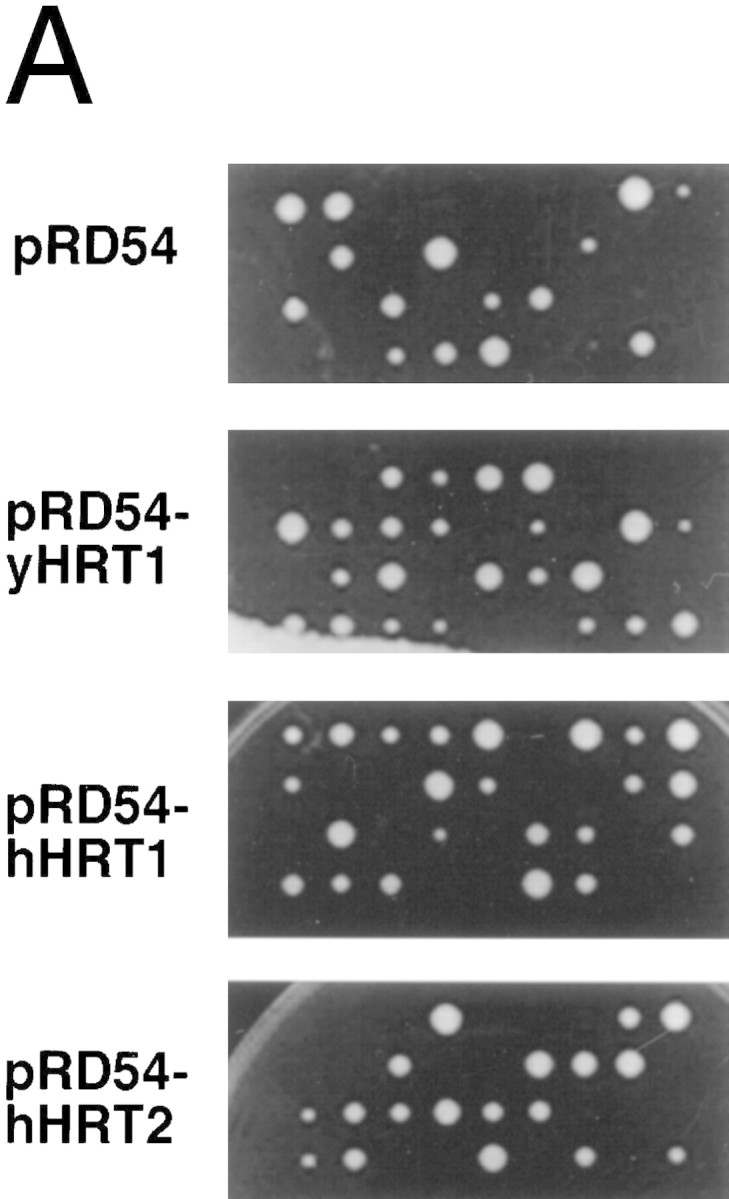
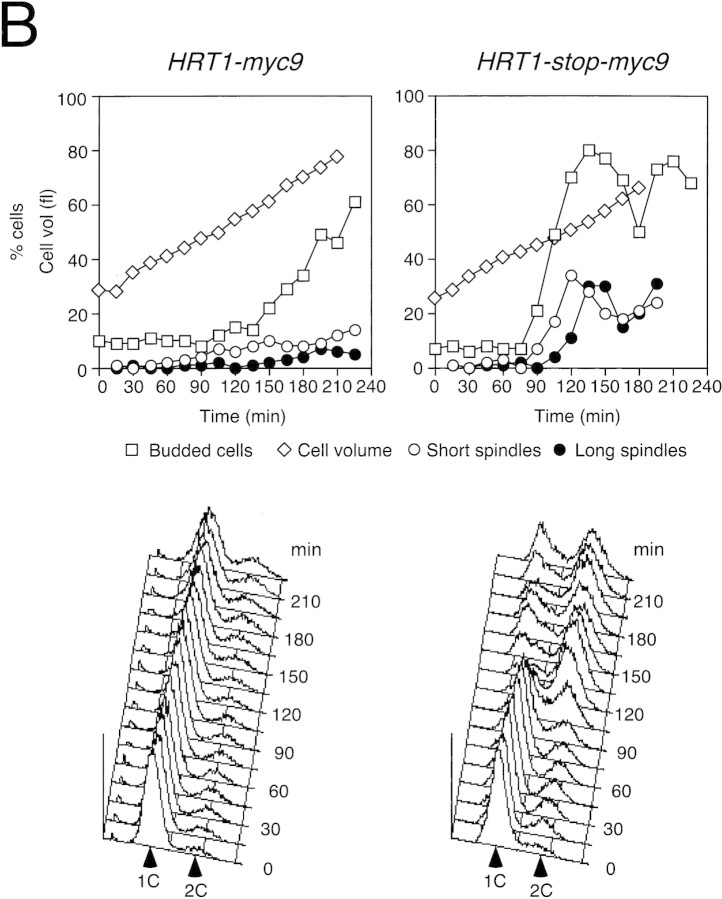
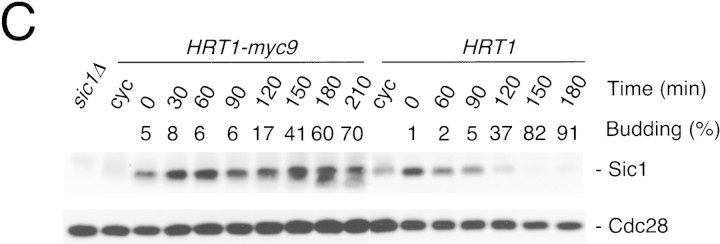
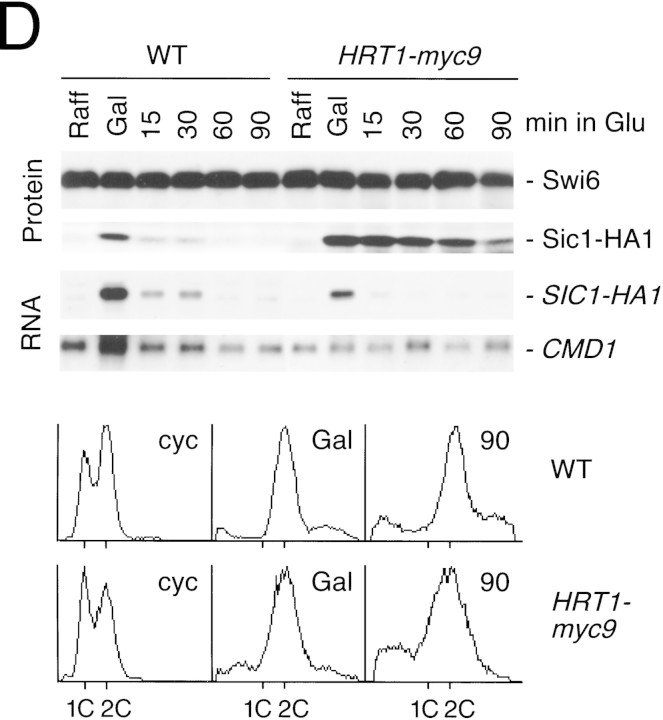
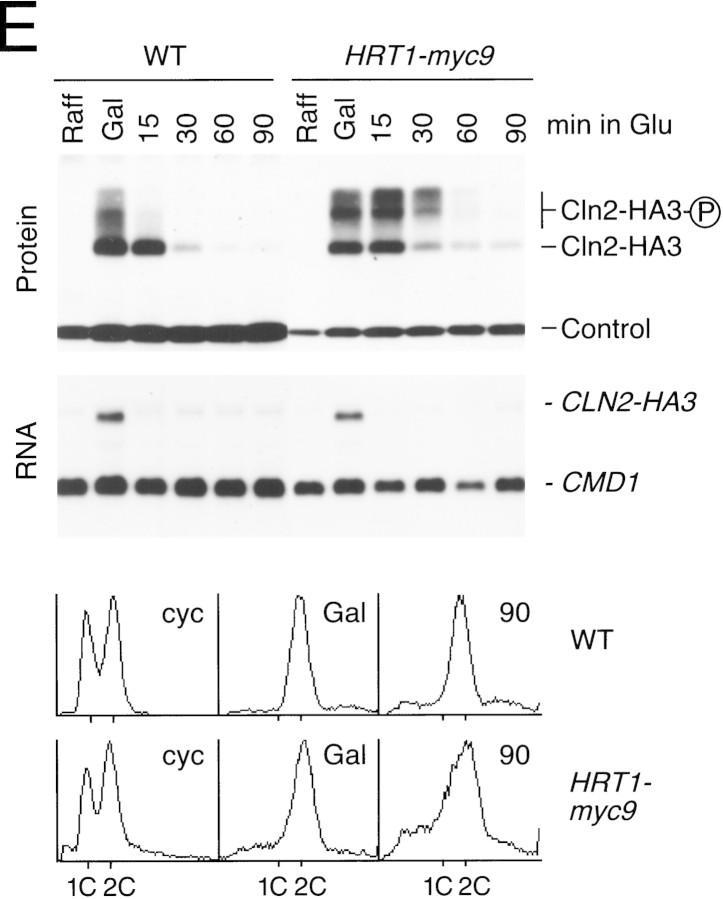
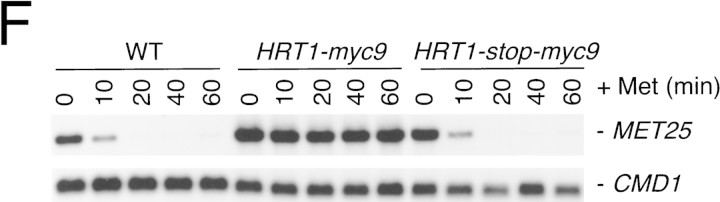
(A) HRT1 is essential. A diploid hrt1::HIS3/HRT1 strain was transformed with pRD54-based plasmids (pRS316 CEN, URA3) containing either yeast HRT1, human HRT1, or human HRT2 under the control of the galactose-inducible GAL1 promoter. Sporulated diploids were dissected on complete galactose medium, followed by incubation at 24°C for 5 days. (B,C) HRT1 is required for both elimination of Sic1 and for S phase. A myc9 epitope cassette was integrated at HRT1 either immediately upstream (HRT1–myc9, left) or downstream (HRT1–stop–myc9, right) of the stop codon. Small G1 cells were isolated by centrifugal elutriation from cultures grown in YPD at 25°C and inoculated into fresh medium at 37°C at time 0. In B, mean cell volume (fl), budding index, formation of mitotic spindles (top panels), and DNA contents (bottom panels) were assessed at the indicated times. In C, budding index, Sic1 levels, and Cdc28 levels were monitored. (D) HRT1 is required for proteolysis of Sic1. HRT1 cells containing five integrated copies of GAL–SIC1–HA1 and HRT1–myc9 GAL–SIC1–HA1 (one copy) cells were grown in YP–raffinose at 25°C (cyc) and arrested in mitosis with nocodazole (Raff). After shifting the cultures to 32°C, expression from the GAL promoter was induced with galactose (Gal) and then repressed by transferring the cells to YP–glucose. Samples were withdrawn at the indicated time points and assayed for: Swi6 and Sic1-HA1 protein levels (top panels); calmodulin and SIC1–HA1 mRNA levels (middle panels); and cellular DNA content (bottom panels). (E) HRT1 is required for rapid degradation of Cln2. Same as D, except CLN2–HA3 expression was induced with galactose at 35°C, and cells were shifted to 37°C upon transfer to YP–glucose. The band labeled control is an unknown polypeptide recognized by 12CA5. (F) HRT1 is required for methionine-dependent repression of MET25 mRNA. Yeast strains indicated were grown in complete medium containing glucose and lacking methionine at 25°C and were then shifted to 37°C for 2 hr. Methionine was added to the medium and samples were withdrawn at the indicated times. MET25 and CMD1 mRNA levels were assayed by Northern blotting.