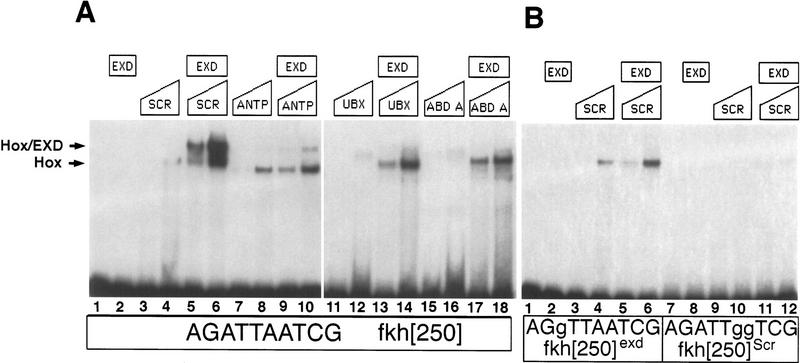
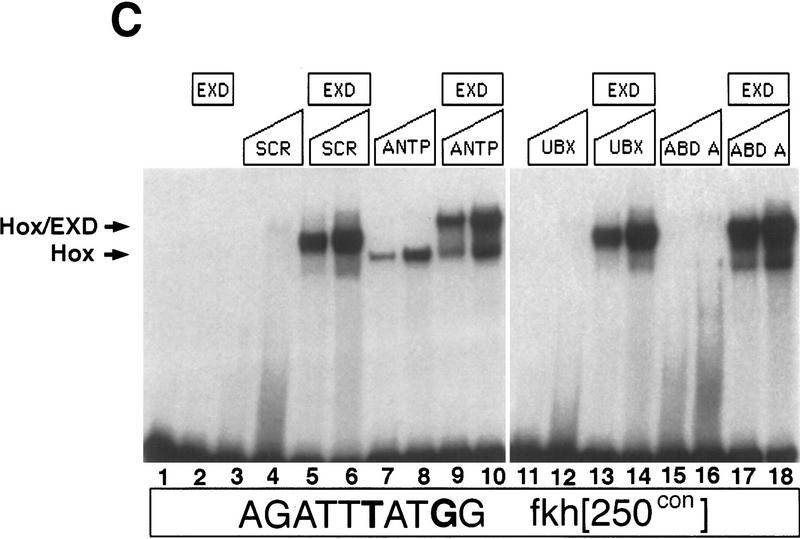
Figure 3.
Hox/Exd-binding preferences of fkh[250] and fkh[250con]. (A) EMSA using the fkh[250] oligo with Hox and Exd proteins as indicated. There is a preference for binding Scr/Exd heterodimers over Antp/Exd, Ubx/Exd, and Abd-A/Exd heterodimers. (B) EMSA with the fkh[250]exd or fkh[250]Scr oligos with Scr and Exd as indicated. Mutation of the Exd half-site (fkh[250]exd) or the Scr half-site (fkh[250]Scr) abolished Scr/Exd complex formation. (C) EMSA with the fkh[250con] oligo with Hox and Exd proteins as indicated. All four heterodimers (Scr/Exd, Antp/Exd, Ubx/Exd, Abd-A/Exd) bound strongly to the fkh[250con] oligonucleotide. The concentrations of all proteins used in C were identical to those in A, and these EMSAs were carried out at the same time.