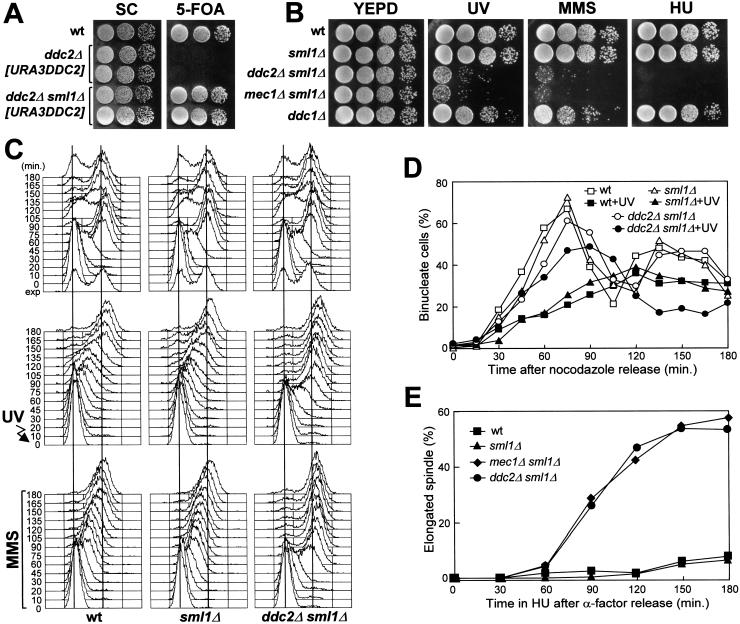
Figure 1.
DNA damage hypersensitivity and checkpoint defects of ddc2Δ sml1Δ cells. Strains were as follows: wild type (K699), ddc2Δ [URA3 DDC2] (YLL275), ddc2Δ sml1Δ [URA3 DDC2] (DMP2995/7A), sml1Δ (YLL488) (Longhese et al. 2000), ddc2Δ sml1Δ (DMP2995/1B), mec1Δ sml1Δ (YLL490) (Longhese et al. 2000), and ddc1Δ (YLL244). (A–B) Serial dilution of YEPD-exponentially growing cell cultures were spotted on SC plates with or without 5-FOA (A) or on YEPD plates with or without MMS (0.005%) or HU (5 mm) (B). YEPD plates were made in duplicate, and one of them was UV-irradiated (30 J/m2) (UV). (C) α-Factor-synchronized cells were released from α-factor at time zero in YEPD (top), were UV-irradiated (40 J/m2) prior to the release in YEPD (middle), or were released in YEPD containing 0.02% MMS (bottom). Samples of untreated, UV- and MMS-treated cell cultures were collected at the indicated times after α-factor release and analyzed by FACS. (D) Cell cultures were arrested with nocodazole and were UV-irradiated (50 J/m2). Cell cycle progression was monitored at the indicated times in unirradiated and UV-irradiated cultures after release from nocodazole, by direct visualization of nuclear division by propidium iodide staining. (E) Cell cultures were arrested in G1 with α-factor and then released at time zero in YEPD containing 200 mm HU. Aliquots of cells were collected at the indicated times and stained with antitubulin antibodies to score for the percentage of cells with elongated spindles by indirect immunofluorescence. FACS analysis of the DNA content and plating for cell survival were carried out concomitantly (see text for details).