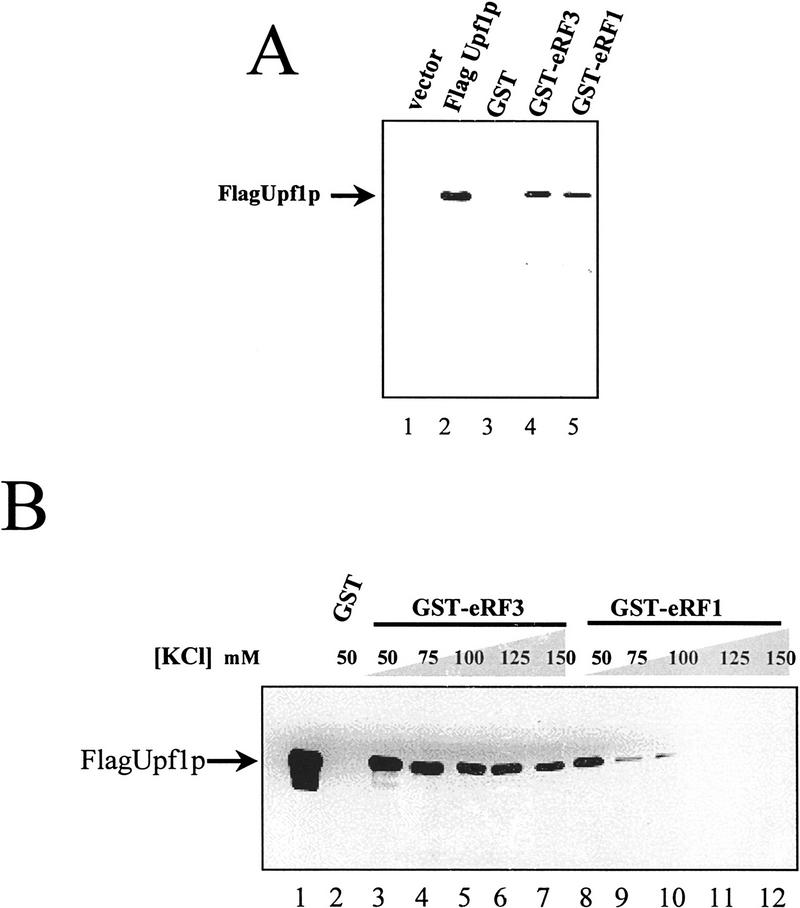
Figure 1.
The yeast Upf1 protein interacts specifically with the peptidyl release factors. (A) GST–eRF1 or GST–eRF3 fusion proteins bind specifically to Upf1p in a yeast extract. Cytoplasmic extracts from a yeast strain BJ3505 transformed with either pG-1 (vector) or pG-1FLAGUPF1 (Flag-Upf1p) were prepared in IBTB and incubated with 30 μl of GST, GST–eRF1, or GST–eRF3 Sepharose–protein complexes. The Sepharose–protein complexes were washed twice in IBTB (see Materials and Methods), resuspended in SDS–polyacrylamide loading buffer, separated on an 8% SDS-polyacrylamide gel, and immunoblotted by use of anti-Flag antibody. (B) Upf1p interacts directly with both eRF1 and eRF3. Upf1p was purified as described previously (Czaplinski et al. 1995). Upf1p (200 ng) was added to 10 μl of GST, GST–eRF1, or GST–eRF3 Sepharose–protein complexes in a total reaction volume of 200 μl in IBTB supplemented with KCl to the final concentration indicated above each lane. After 1 hr at 4°C, Sepharose–protein complexes were washed for 3 min with 1 ml of IBTB supplemented with KCl to the final concentration indicated above each lane. The purified Sepharose–protein complexes were resuspended in SDS-polyacrylamide loading buffer and separated on a 7.5% SDS-polyacrylamide gel and immunoblotted as in A.