Figure 8.
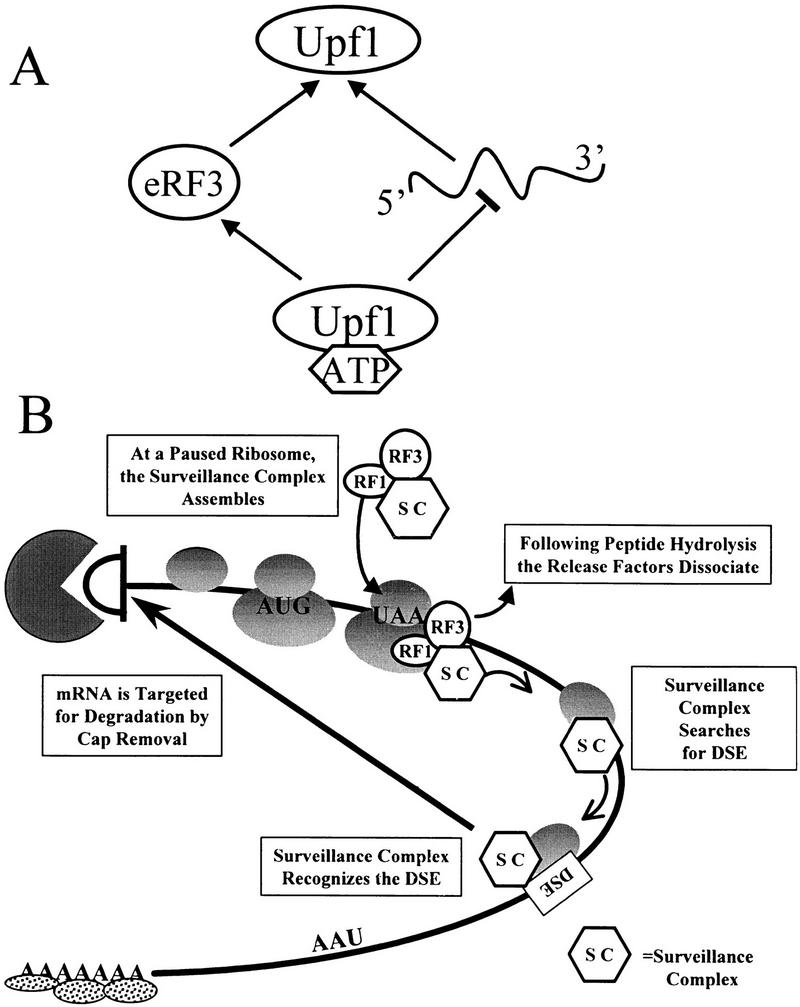
Model for Upf1 function in mRNA surveillance. (A) Modulation of RNA binding enhances interaction of Upf1 with peptidyl release factors. ATP binding to Upf1p decreases the affinity of Upf1 for RNA. Because RNA and eRF3 compete for binding to Upf1, interaction with eRF3 is favored. (B) A model for mRNA surveillance. Interaction of Upf1p with peptidyl release factors assembles an mRNA surveillance complex at a termination event. This interaction prevents Upf1 from binding RNA and hydrolyzing ATP, and enhances translation termination. Following peptide hydrolysis, the release factors dissociate from the ribosome, activating the Upf1p helicase activity. The surveillance complex then scans 3′ of the termination codon for a DSE. Interaction of the surveillance complex with the DSE signals that premature translation termination has occurred and the mRNA is then decapped and degraded by the Dcp1p and Xrn1p exoribonuclease, respectively.
