Figure 3.
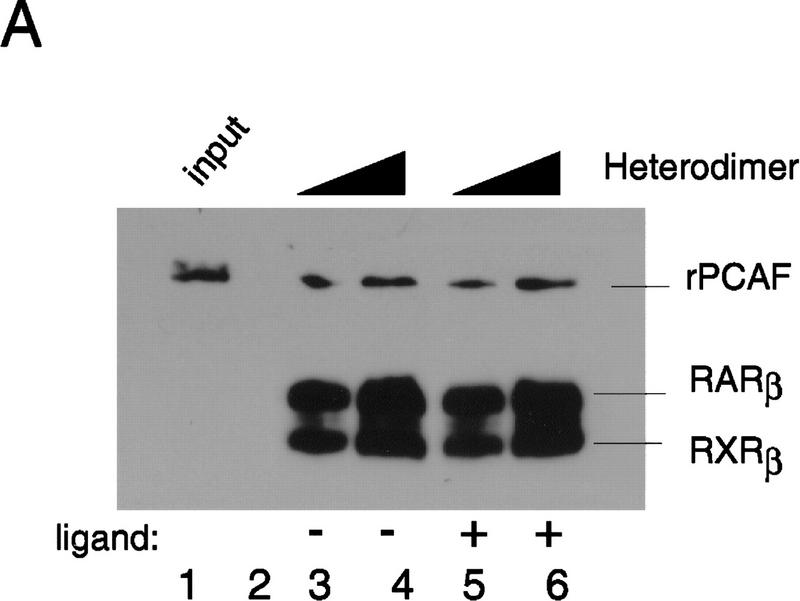
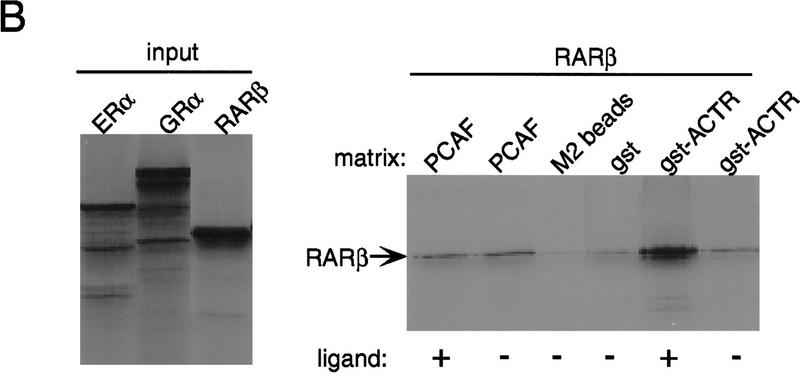
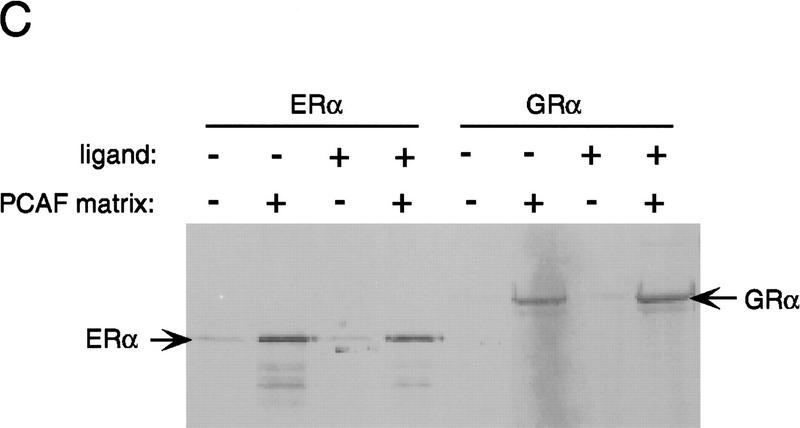
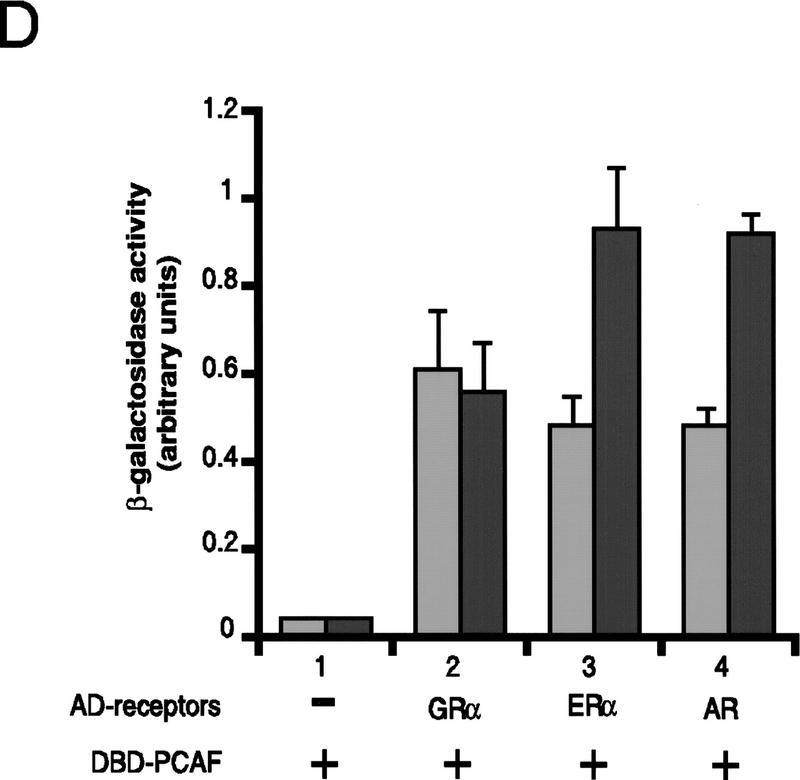
Binding of PCAF to various nuclear hormone receptors in vitro. (A) Direct binding of rPCAF to RXR–RAR heterodimers. rPCAF (2 pmoles, 200 ng) was incubated with increasing amounts of heterodimers (0.5 μg, lanes 3,5 or 1 μg, lanes 4,6) bound to the RARE-conjugated beads in the presence (+) or absence (−) of 1 μm 9-cis RA. Binding of rPCAF was detected by immunoblot analysis with anti-M2-Flag antibody. (Lane 1) 20 ng of rPCAF as input; (lane 2) eluates from RARE-conjugated beads without receptor. (B) Binding of RARβ to rPCAF. Binding of [35S]methionine-labeled RARβ was tested with rPCAF immobilized to the M2 antibody matrix or matrix alone (M2), GST beads, GST–ACTR in the presence (+) or absence (−) of 1 μm 9-cis RA. (Right) Radiolabeled receptor input (30%) tested in B and C. (C) Binding of 35S-labeled ERα or GRα to rPCAF immobilized to the M2 antibody matrix was tested in the presence (+) or absence (−) of 1 μm β-estradiol (for ERα) or 1 μm dexamethasone (for GR). (D) PCAF–receptor interactions detected by yeast two–hybrid assays. Yeast strain Y190 was transformed with indicated GAL4-fusion plasmids. The liquid β-gal assays were performed for the transformants, which were grown in the presence or absence of corresponding hormones (10 nm deoxycorticosterone for GR, 100 nm β-estradiol for ER, and 100 nm dihydrotestosterone for AR). Results represent the average of three independent yeast transformants ± s.d.