Abstract
We describe an efficient homogeneous ruthenium catalyst for the dehydrogenation of ammonia borane (AB). This catalyst liberates greater than 2 equivalents of H2 and up to 4.6 system wt% H2 from concentrated AB suspensions under air. Importantly, this catalyst is robust, delivering several cycles of dehydrogenation at high [AB] without loss of catalytic activity, even with exposure to air and water.
Catalytic dehydrogenation of ammonia borane is an active research area because AB is a potentially useful hydrogen storage medium for transportation.i This is because of its high hydrogen density (19.6 wt%), ability to release H2 under mild conditions, both thermalii and catalytic, and desirable physical properties. Though catalytic hydrolysis is well known and very efficient for H2 production from AB,ib anhydrous dehydrogenation can enable a more efficient fuel cycle. This is because hydrolysis reactions form stoichiometric quantities of ammonia, a hydrogen fuel cell poison, and strong B–O bonds, which preclude an efficient regeneration scheme.iii Several heterogeneousiv and homogeneousv transition metal catalysts are active for AB dehydrogenation, but these are limited by protic and oxidative decomposition in air, low extent of H2 release (≤2 equiv.), uncontrolled rate of H2 release, or production of unwanted products such as ammonia (NH3) or borazine (N3B3H6), which are poisonous to fuel cells. Additionally, many are unviable because the catalyst loading is high or the catalyst is not reusable. We report here a system that is long-lived (TON > 5000), functions under air, and dehydrogenates AB through > 2 equiv. H2. These characteristics make it a leading candidate for use in a commercial H2 storage system.
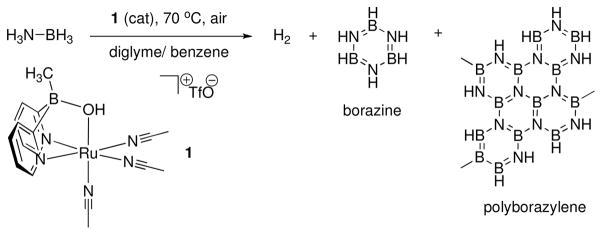
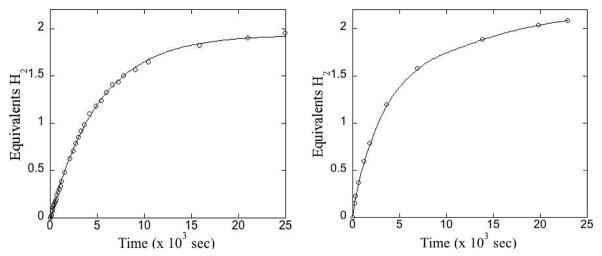
Catalyst (1)vi liberates H2 from AB under air in diglyme solutions (Scheme 1). To determine the efficiency of AB dehydrogenation with 1, we treated a 0.42 M AB solution with 5.0 mol% 1 at 70 °C in a Schlenk flask under air and monitored the production of H2 using a eudiometer. The system produced 1.94 equiv. of H2 in 7 hours, Figure 1, left. Added metallic mercury did not inhibit the reaction under these conditions, which indicates homogeneous catalysis.vii We also examined more practical, highly concentrated suspension conditions. A slurry of AB and tetraglyme (100.0 mg AB, 202.6 mg tetraglyme, 5.8 system wt% stored H2) was treated with 2.0 mol% 1 at 70 °C yielding 2.0 equiv. of H2 in 4 hours and a total of 2.2 equiv. (TON = 110) upon completion of the reaction. This corresponds to the release of 4.2 wt% H2. In contrast, uncatalyzed AB dehydrogenationviii liberates only 1.6 equiv. of H2 at 70 °C at a rate ca. 2.5 × slower under our reaction conditions (see Supporting Information).
Scheme 1.
Dehydrogenation of ammonia borane to produce hydrogen, borazine, and polyborazylene
Figure 1.
Left: H2 production from AB with 5.0 mol% 1 under air at 70 °C in 11:1 diglyme/benzene solution. Right: H2 production from AB in the presence of 2.0 mol% 1 in tetraglyme under air at 70 °C at high [AB]. H2 release = 4.2 system wt%.
This catalyst system is also remarkably reusable. Data for successive runs at 2.0 mol% loading (70 °C) indicate that catalysis is efficient through four ca. 6 hour runs at high [AB], although the reaction slurry becomes viscous. To help alleviate this situation we added 0.2 mL tetraglyme while recharging the reactor with AB for subsequent runs. In this way we ran four successive runs in a single reactor. The rates for each were similar, and they released 2.2, 2.1, 2.3, and 2.2 equiv. H2, respectively, for a total TON of 440. With a catalyst loading of 0.1 mol% we have observed a TON of ca. 5700 over three runs and liberation of up to 4.6 wt% H2.ix For comparison, Fagnou reported a ruthenium catalyst that could release up to 1.0 system wt% from AB at room temperature and up to 3.6 system wt% H2 from AB/MeNH2BH3 mixtures at 50 °C.vb This is the best previously-published weight-content H2 release for a homogeneous catalytic system dehydrogenating an amine-borane.
Our system works well when open to air, which is beneficial for commercialization: a system stable to air and water provides practical advantages over a more sensitive one. Measurement of H2 produced in eudiometry studies in air and under N2 were identical under concentrated AB conditions. To further quantify this effect, we compared rate constants (kobs) for [AB] consumption obtained for reactions conducted under nitrogen and air. In this experiment 2.5 mol% 1 was prepared in diglyme/benzene-d6 in the glovebox and sonicated open to air for 1 h prior to addition of AB to ensure removal of the N2 environment and establish atmospheric O2 levels in the NMR tube. The kobs values at 70 °C were 2.65(9) × 10−4 s−1 in air and 2.54(9) × 10−4 s−1 in N2. Thus, we see neither inhibition nor acceleration of catalysis. This suggests that at ambient levels of O2, catalysis is not shut down by decomposition of the active species or accelerated by formation of a more reactive, higher valent ruthenium oxo complex.
We have probed the mechanism of AB dehydrogenation with 1 by 11B NMR in dilute diglyme solutions. Unlike Shvo’s catalyst,ve 1 demonstrates first order kinetics in [AB] through 3 half lives as observed by disappearance of the AB resonance in the 11B NMR spectrum. Similar to others’ observations, the intermediates we see in these reactions are branched and unbranched cyclotri-borazane, aminoborane oligomers, borazine, and polyborazylene (PB),ia,d,viii with the latter two being the major products upon completion. As is the case for Shvo’s catalyst,ve we do not observe formation of insoluble B, N oligomers in this system.
The appearance of polyborazylene at early times in reactions with 1 is encouraging, whereas none is seen with Shvo conditions. Formation of PB is a feature of extensive dehydrogenation, indicating production of greater than 2 equiv. of H2, and has been proposed to occur by borazine cross-linking in transition metal catalyzed systems. However, in contrast to the (NHC)Ni systems in which borazine was quickly consumed,vd [borazine] increases over time in reactions of 1 and is not consumed late in the reaction.x This suggests that catalytic borazine cross-linking is not the mechanism for PB formation in this system. We suspect that the operative mechanism involves reaction of borazine with AB or one of its early dehydrogenation products. Efficient, catalytic borazine cross-linking has not yet been demonstrated in the absence of other B, N materials.xi
Rate data (kobs, 70 °C) collected by 11B NMR are pseudo-first order in AB. A catalyst order study was conducted by comparing these kobs values measured at varying concentrations of 1. The data give a linear ln(kobs) vs. ln([1]) plot with a slope of 0.56, which indicates the presence of a dinuclear (Ru)2 intermediate,xii although we have not observed this species. Possible structures include those with a B–O–B bridge, bridging hydride, or a product of AB dehydrogenation serving as bridging moiety. Along these lines, we have not observed a persistent metal hydride, which argues against a Ru–H moiety as the resting state of the catalyst. This is in line with observations of the (NHC)Ni systems, vd but contrasts our ownve and others’ findings for ruthenium vb,c and iridiumva catalysts.
We suspect that 1 might be analogous to several known catalysts that interact with the polarized B–H and N–H bonds of AB concurrently in a bifunctional transition state.vb–f,xiii We probed this by recording deuterium isotope effects for isotopologues of AB.xiv kH/kD values for the B–H and N–H bonds are 1.22(14) and 1.58(9), respectively. A combined isotope effect measured using D3N–BD3 (kNHBH/kNDBD) is 1.67(18). Thus, the product of the two independently measured isotope effects (1.92(25)) is within error of the measured double isotope effect, which is consistent with a concerted, asynchronous transition state in the rate-determining step. However, this conclusion is tentative since we cannot be certain whether the observed isotope effects involve H2 transfer from AB to catalyst or multiple steps, as in the (NHC)Ni system. xiiia
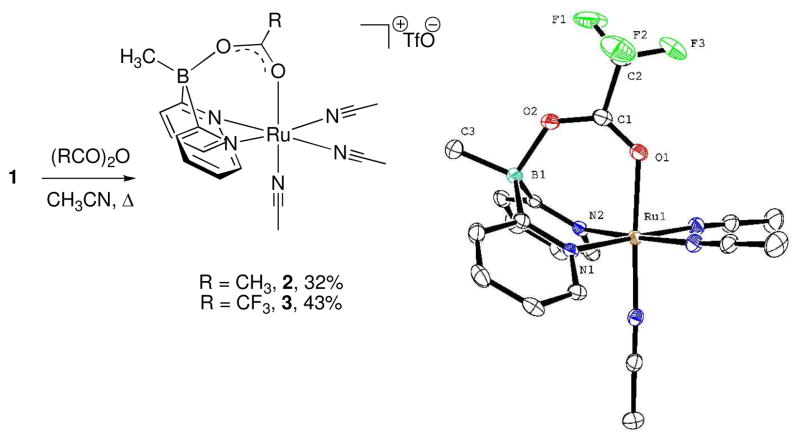
Other mechanistic scenarios have the bridging hydroxyl group of 1 donating H+ to the solution, thus initiating an acid catalyzed reaction,xv or the so-formed oxide bridge providing an internal base in a bifunctional mechanism. To probe these we synthesized bridging carboxylate complexes 2 and 3 (Scheme 2, left), which are devoid of the protic functionality. The crystal structure of 3 (Scheme 2, right)xvi shows an unprecedented Ru–B μ2-kO:kO′ bonding mode for the trifluoroacetate wherein the boron distorts from its tetrahedral geometry to a trigonal pyramidal structure. This behavior could be rationalized by unfavorable steric interactions between the bridging carboxylate and other ligands in the unobserved μ2-κO:κO (single bridging oxygen) mode. We suspect the same for 2.
Scheme 2. Synthesis of bridging carboxylate complexes 2 and 3.
Above: Synthesis. Right: ORTEP plot of 3 with 50% probability ellipsoids. Hydrogen atoms and triflate counterion omitted for clarity.
Surprisingly, 5.0 mol% solutions of 1 and 2 have the same rate (kobs = 3.74(7) × 10−4 s−1, 3.72(10) × 10−4 s−1, respectively, at 70 °C) for AB consumption. This disfavors an H+-catalyzed mechanism. To interrogate the potential role of boron as a Lewis acid, we attempted AB dehydrogenation with complex 3, which has different electronic characteristics than 1 or 2. This gives faster catalysis: 2.5 mol% solutions of 1 and 3 give rates of kobs = 2.66(12) × 10−4 s−1 and 5.77(12) × 10−4 s−1, respectively, for AB consumption at 70 °C. Thus, the occupancy of the bridging coordination site between Ru and B has an important influence on dehydrogenation rate.
In multiple solvents we have observed that the acetate and trifluoroacetate complexes hydrolyze completely in the presence of trace water to reform bridging hydroxide complex 1 and the corresponding carboxylic acid, which indicates a thermodynamic preference for the Ru(OH)B bridge. Adding water (ca. 6 equiv.) to a sample of 3 in tetrahydrofuran-d8 reveals that hydrolysis has a half-life of 6–7 hours at room temperature, thus demonstrating the lability of the carboxylate ligand. Further, a methylene chloride-d2 solution of 3 and 1 equiv. TFA-d1 shows coalescence of the bound and free TFA signals at 50 °C as monitored by 19F NMR: two fluorine resonances (δ = −75.38 (bound) and δ = −75.25 (free) at 25°C) coalesce to one (δ = −75.51). This observation indicates that exchange is occurring rapidly on the NMR timescale. Thus, the bridging trifluoroacetate appears to be sufficiently labile to allow boron and ruthenium to participate in catalysis, but these data do not rule out participation of the acetate and trifluoroacetate in the mechanism of AB dehydrogenation by 2 and 3, as has been documented in systems for hydrocarbon C–H activation.xvii
Importantly, dehydrogenation is not efficient in the absence of the borate ligand. Catalyst 1’s non-ligated synthetic precursor, [(cym)RuCl2]2 (4), does not participate in efficient catalysis; these reactions precipitate metallic material under our experimental conditions. 11B NMR data for catalysis with 4 reveal a rate of AB consumption that is only ca. 2× lower than that of 1, but H2 production from this system is limited to 1 equiv. and it is not reusable. Additionally, added metallic mercury significantly attenuates the production of H2 with 4. Full kinetics data for AB dehydrogenation with 4, Na[(2-py)2BMe2], and [((2-py)2BMe2)RuCl(cym)] (5) are described in Supporting Information.
In conclusion, we have demonstrated an efficient and robust catalyst for highly productive ammonia borane dehydrogenation. This catalyst liberates up to 4.6 wt% H2 from AB suspensions and is resistant to deactivation in air, which makes it one of the most appealing homogeneous transition metal catalysts designed to date. Its longevity at low catalyst loadings (TON up to 5700) and its air stability are unprecedented in transition metal catalyzed AB dehydrogenation. Initial mechanistic investigations based on (1) isotope effects and (2) the relative reaction rates of 2 and 3, suggest that dual-site cooperativity could be operative. This mechanistic insight might lead to the development of more efficient systems for AB dehydrogenation. Ongoing work in our laboratory involves uncovering the detailed roles of the boron and ruthenium centers in AB dehydrogenation and the application of dual-site catalysis to more general hydride manipulation reactions.
Supplementary Material
Acknowledgments
We thank the National Science Foundation (CHE-1054910), University of Southern California, Loker Hydrocarbon Research Institute, and Hydrocarbon Research Foundation for research support and the NSF (DBI-0821671, CHE-0840366) and NIH (S10-RR25432) for NMR spectrometers. We thank Ralf Haiges for the X-ray study of 3.
Footnotes
SUPPORTING INFORMATION. Full experimental procedures, characterization of complexes 2 and 3, and full kinetics data for ammonia borane dehydrogenation with 1–5 and Na[(2-py)2BMe2], and a comment on the rate dependence on [EtOH]. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (i).Staubitz A, Robertson APM, Manners I. Chem Rev. 2010;110:4079–4124. doi: 10.1021/cr100088b.Stephens FH, Pons V, Baker RT. Dalton Trans. 2007:2613–2626. doi: 10.1039/b703053c.Marder TB. Angew Chem Int Ed. 2007;46:8116–8118. doi: 10.1002/anie.200703150.Hamilton CW, Baker RT, Staubitz A, Manners I. Chem Soc Rev. 2009;38:279–293. doi: 10.1039/b800312m.and references therein
- (ii).(a) Baitalow F, Bauman J, Wolf G, Jaenicke-Rossler K, Leitner G. Thermochim Acta. 2002;391:159–168. [Google Scholar]; (b) Wolf G, Baumann J, Baitalow F, Hoffmann FP. Thermochim Acta. 2000;343:19–25. [Google Scholar]; (c) Wang JS, Geanangel RA. Inorg Chim Acta. 1988;148:185–190. [Google Scholar]; (d) Bluhm ME, Bradley MG, Butterick R, III, Kusari U, Sneddon LG. J Am Chem Soc. 2006;128:7748–7749. doi: 10.1021/ja062085v. [DOI] [PubMed] [Google Scholar]; (e) Rassat SD, Aardahl CL, Autrey T, Smith RS. Energy Fuels. 2010;24:2596–2606. [Google Scholar]
- (iii).(a) Davis BL, Dixon DA, Garner EB, Gordon JC, Matus MH, Scott B. Angew Chem Int Ed. 2009;48:6812–6816. doi: 10.1002/anie.200900680. [DOI] [PubMed] [Google Scholar]; (b) Sutton AD, Burrell AK, Dixon DA, Garner EB, III, Gordon JC, Nakagawa T, Ott KC, Robinson JP, Vasiliu M. Science. 2011;331:1426–1429. doi: 10.1126/science.1199003. [DOI] [PubMed] [Google Scholar]
- (iv).(a) Jaska CA, Temple K, Lough AJ, Manners I. J Am Chem Soc. 2003;125:9424–9434. doi: 10.1021/ja030160l. [DOI] [PubMed] [Google Scholar]; (b) Jaska CA, Manners I. J Am Chem Soc. 2004;126:1334–1335. doi: 10.1021/ja039162w. [DOI] [PubMed] [Google Scholar]; (c) Shrestha RP, Diyabalanage HVK, Semelsberger TA, Ott KC, Burrell AK. Int J Hydrogen Energy. 2009;34:2616–2621. [Google Scholar]
- (v).(a) Denney MC, Pons V, Hebden TJ, Heinekey M, Goldberg KI. J Am Chem Soc. 2006;128:12048–12049. doi: 10.1021/ja062419g. [DOI] [PubMed] [Google Scholar]; (b) Blaquiere N, Diallo-Garcia S, Gorelsky I, Black A, Fagnou K. J Am Chem Soc. 2008;130:14034–14035. doi: 10.1021/ja804235t. [DOI] [PubMed] [Google Scholar]; (c) Käβ M, Friedrich A, Drees M, Schneider S. Angew Chem Int Ed. 2009;48:905–907. doi: 10.1002/anie.200805108. [DOI] [PubMed] [Google Scholar]; (d) Keaton RJ, Blacquiere M, Baker RT. J Am Chem Soc. 2007;129:1844–1845. doi: 10.1021/ja066860i. [DOI] [PubMed] [Google Scholar]; (e) Conley BL, Williams TJ. Chem Comm. 2010;46:4815–4817. doi: 10.1039/c003157g. [DOI] [PubMed] [Google Scholar]; (f) Chapman AM, Haddow MF, Wass DF. J Am Chem Soc. 2011;133:8826–8829. doi: 10.1021/ja201989c. [DOI] [PubMed] [Google Scholar]; (g) Wright WRH, Berkeley ER, Alden LR, Baker RT, Sneddon LG. Chem Commun. 2011;47:3177–3179. doi: 10.1039/c0cc05408a. [DOI] [PubMed] [Google Scholar]; (h) Kim S-K, Han W-S, Kim T-J, Kim T-Y, Nam SW, Mitoraj M, Piekoś Ł, Michalak A, Hwang S-J, Kang SO. J Am Chem Soc. 2010;132:9954–9955. doi: 10.1021/ja101685u. [DOI] [PubMed] [Google Scholar]
- (vi).Conley BL, Williams TJ. J Am Chem Soc. 2010;132:1764–1765. doi: 10.1021/ja909858a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (vii).Anton DR, Crabtree RH. Organometallics. 1983;2:855–859.Widegren JA, Finke RG. J Mol Cat A: Chem. 2003;198:317–341.The mercury drop test is commonly invoked to provide positive evidence for homogeneous catalysis when a reaction is not inhibited upon its addition. As pointed out by a reviewer and thoroughly discussed in (b) of this reference, a Hg drop test is not definitive. Some transition metals are more difficult to amalgamate (i.e. Ru) and care should be taken in analysis when using precursors based on these metals. More informative to the point of homogeneous vs. heterogeneous catalysis, though not conclusive, kinetic behavior, solution turbidity, and order in precatalyst. In our case the sum of our results suggests homogeneous behavior
- (viii).Shaw WJ, Linehan JC, Szymczak NK, Heldebrant DJ, Yonker C, Camaioni DM, Baker RT, Autrey T. Angew Chem Int Ed. 2008;47:7493–7496. doi: 10.1002/anie.200802100. [DOI] [PubMed] [Google Scholar]
- (ix).System wt% H2 production was calculated over four cycles (combined) using the following formula: [(Theoretical wt yield of H2)/(Total wt AB + wt of solvent + wt of catalyst)] × (Total eq H2 liberated/Theoretical eq H2). For the four cycles at 2.0 mol% this is: [(78.4 mg)/(400.0 mg + 810.4 mg + 38.0 mg)] × (8.8 eq/12.0 eq) × 100% = 4.6 system wt%.
- (x).Slow thermal cross-linking is observed as described by Fazen PJ, Remsen EE, Beck JS, Carroll PJ, McGhie AR, Sneddon LG. Chem Mater. 1995;7:1942–1956.
- (xi).Baker et. al reported extensive dehydrogenation of AB to polyborazylene (Ref. 4d), but control reactions with added borazine and preformed catalyst revealed no cross-linking. We have also failed to observe cross-linking of borazine with 1 under the reaction conditions reported herein.
- (xii).Espenson J. Chemical Kinetics and Reaction Mechanisms. 2. McGraw-Hill; 2002. pp. 77–82. [Google Scholar]
- (xiii).(a) Zimmerman PM, Paul A, Zhang Z, Musgrave CB. Angew Chem Int Ed. 2009;48:2201–2205. doi: 10.1002/anie.200803211. [DOI] [PubMed] [Google Scholar]; (b) Yang X, Hall MB. J Am Chem Soc. 2008;130:1798–1799. doi: 10.1021/ja0751328. [DOI] [PubMed] [Google Scholar]
- (xiv).Hu MG, Van Paasschen JM, Geanangel RA. J Inorg Nucl Chem. 1997;39:2147–2150. [Google Scholar]
- (xv).Stephens FH, Baker RT, Matus MH, Grant DJ, Dixon DA. Angew Chem Int Ed. 2007;46:746–749. doi: 10.1002/anie.200603285. [DOI] [PubMed] [Google Scholar]
- (xvi).CCDC 830936 contains supplementary crystallographic data for compound 3. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- (xvii).(a) Ziatdinov VR, Oxgaard J, Mironov OA, Young KJH, Goddard WA, III, Periana RA. J Am Chem Soc. 2006;128:7404–7405. doi: 10.1021/ja060973k. [DOI] [PubMed] [Google Scholar]; (b) Davies DL, Donald SMA, Macgregor SA. J Am Chem Soc. 2005;127:13754–13755. doi: 10.1021/ja052047w. [DOI] [PubMed] [Google Scholar]; (c) Davies DL, Donald SMA, Al-Duaji O, Macgregor SA, Pölleth M. J Am Chem Soc. 2006;128:4210–4211. doi: 10.1021/ja060173+. [DOI] [PubMed] [Google Scholar]; (d) Bischof SM, Ess DH, Meier SK, Oxgaard J, Nielsen RJ, Bhalla G, Goddard WA, III, Periana RA. Organometallics. 2010;29:742–756. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.