Figure 10.
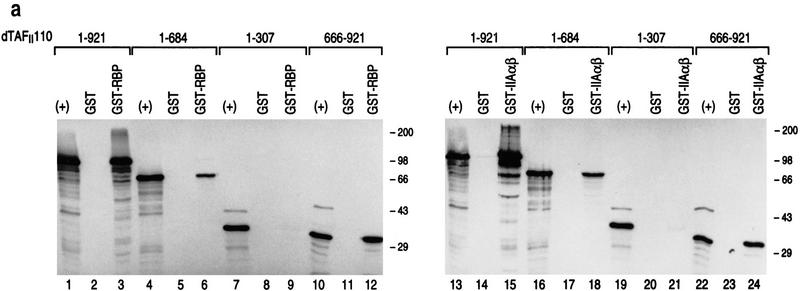
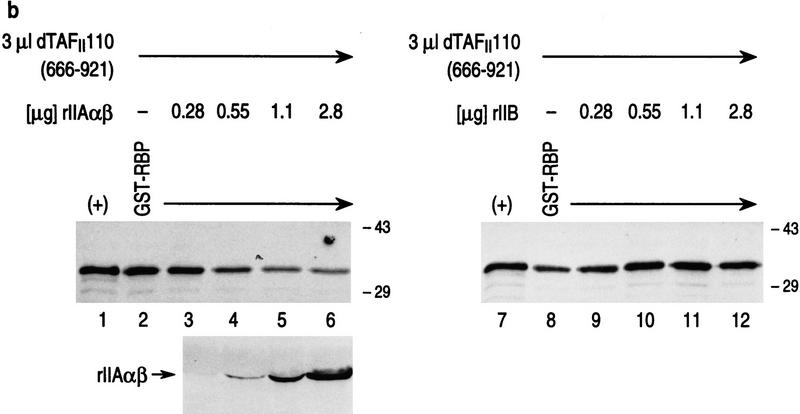
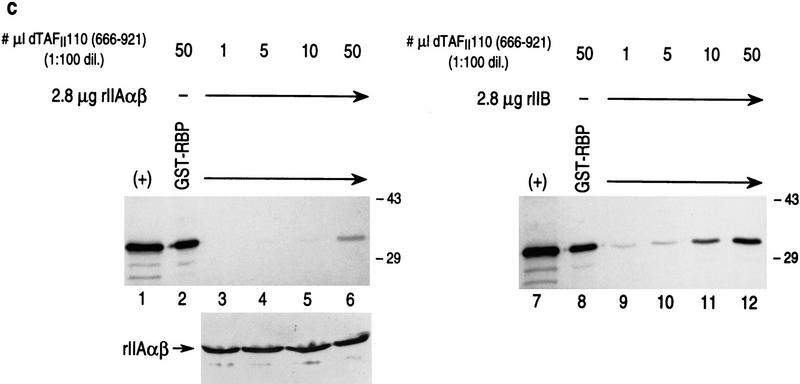
Both RBP and TFIIA α,β interact specifically with the carboxy-terminal domain of dTAFII110, which is inhibited from interaction when RBP complexes with TFIIA α,β. (a) GST pull-down assays with different 35S-labeled dTAFII110 truncated proteins translated in vitro, as indicated at the top of the lanes. Analyses using either GST or GST–RBP (lanes 1–12) and either GST or GST–IIAαβ (lanes 13–24) are shown. The first lane of each set of three shows 10% of the input radiolabeled truncated dTAFII110 examined, the second lane shows the pull-down result with GST, and the third lane shows the pull-down result with either GST–RBP or GST–IIAαβ, as indicated. (b) GST pull-down experiments with GST–RBP in the absence or presence of increasing amounts of either TFIIA α,β or TFIIB, as indicated, and subsequent addition of 3 μl of 35S-labeled dTAFII110 truncation protein containing amino acids 666–921, as indicated. A Western blot analysis of TFIIA α,β interaction with GST–RBP as a function of the addition of increasing amounts of TFIIA α,β is also shown. (+) 10% of the input protein. (c) Similar analysis as performed in b, but with the highest level of TFIIA α, β or TFIIB shown in b and increasing amounts of 35S-labeled dTAFII110 truncated protein containing amino acids 666–921, as indicated. A Western blot analysis of TFIIA α,β interaction with GST–RBP under these conditions is also shown.