Figure 5.
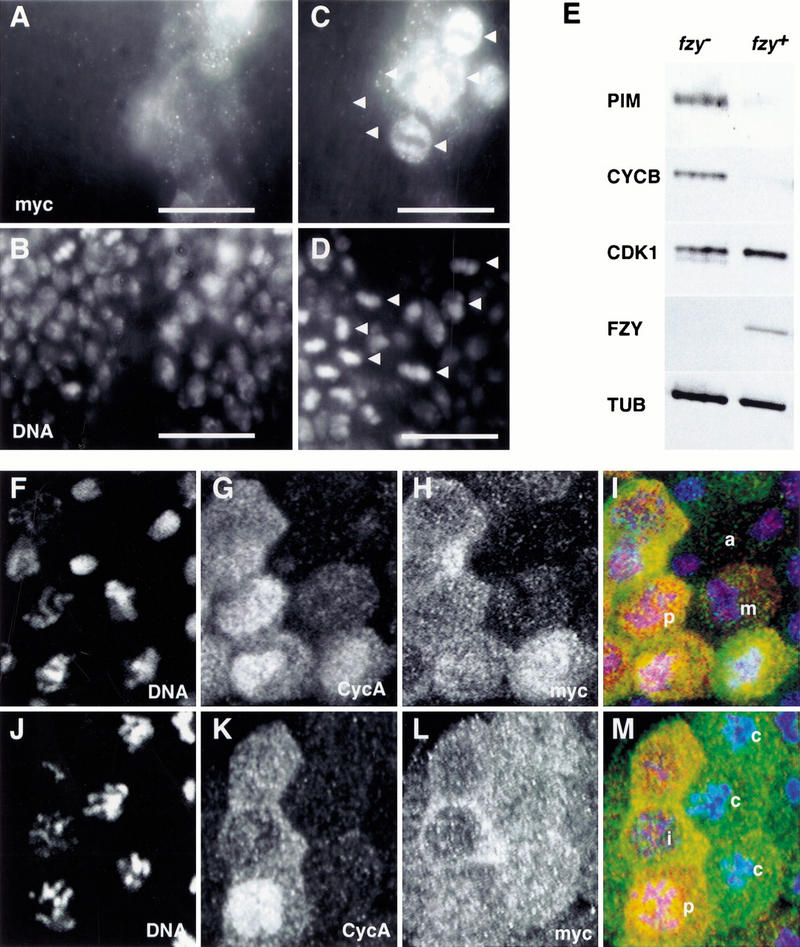
PIM persists during the mitotic arrest caused by colcemid or lack of Fizzy. (A–D) prd–GAL4 was used to express one UAS–pim–myc copy in fizzy mutant embryos. Embryos were fixed at a stage where mitosis 16 is completed during wild-type development and labeled with anti-myc (A,C), a DNA stain (B,D), and anti-β-galactosidase for the identification of fizzy homozygotes (data not shown). High magnification views of the dorsal epidermis (A,B) illustrate that one UAS–pim–myc copy is sufficient to inhibit sister chromatid separation during mitosis 16 in fizzy mutants, leading to the reduced nuclear density in the prd–GAL4-expressing regions (white bar). High magnification views of the ventral epidermis (C,D) illustrate the persistence of PIM–myc in cells arrested in metaphase 16 because of lack of Fizzy (arrowheads), leading to the intense anti-myc labeling in the arrested cells within the prd–GAL4-expressing region (white bar). (E) Progeny from fizzy/CyO parents was aged to the stage where mitosis 16 is completed during wild-type development. fizzy homozygous embryos (fzy−) were sorted from sibling embryos (fzy+) and analyzed by immunoblotting with antibodies against PIM (PIM), Cyclin B (CYCB), Cdk1 (CDK1), FZY (FZY), and tubulin (TUB). (F–M) nos–GAL4–GCN4–bcd3′ UTR was used to express PIM with carboxy-terminal myc epitopes (UAS–pim–myc). Embryos at the stage of mitosis 14 were permeabilized and incubated for 25 min either in the absence (F–I) or presence (J–M) of colcemid before fixation and labeling with antibodies against the myc epitope (myc; H,L), Cyclin A (CycA; G,K) and a DNA stain (DNA; F,J). The merged panels (I,M) show labeling of DNA in blue, Cyclin A in red, and PIM–myc in green. (i) Interphase; (p) prophase; (m) metaphase; (a) anaphase; (c) colcemid-arrested cells.