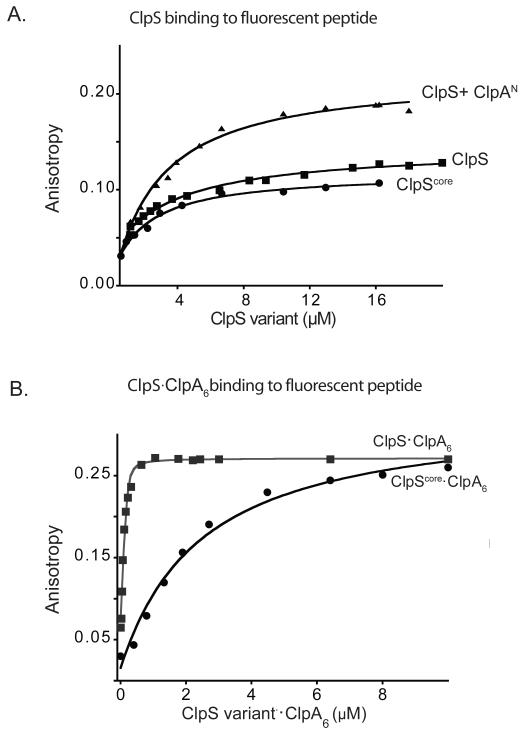
Figure 2. N-end-rule degrons bind more tightly to the ClpS-ClpA6 complex.
A) A fluorescent N-end-rule peptide (LLYVQRDSKEC-fl; 200 nM) was bound with similar affinities (KD ~3 μM) by ClpS, by ClpScore, and by ClpS in complex with the ClpA N domain, as assayed by changes in anisotropy. The molecular weights and maximum anisotropies of each complex differ.
B) Increasing concentrations of 1:1 molar mixtures of ClpA6 and ClpS or ClpScore were titrated against the LLYVQRDSKEC-fl peptide (100 nM). The ClpS-ClpA6 complex bound more tightly (Kapp = 42 ± 6 nM) than the ClpScore-ClpA6 complex (Kapp = 1.5 ± 0.25 μM), demonstrating that the ClpS NTE is required for affinity enhancement. Assays contained 4 mM ATPγS to promote ClpA hexamer formation.