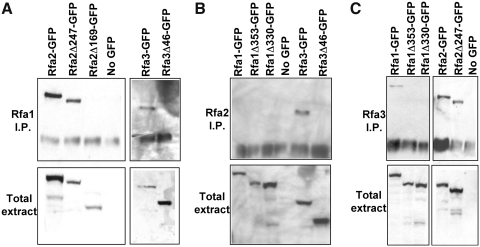
FIG. 5.
Truncated Rfa-GFP fusions that fail to enter the nucleus also fail to bind other members of the RPA complex. Whole cell protein extracts containing one Rfa protein fused to protein A (PrA) and another linked to GFP were immunoprecipitated using IgG-sepharose beads and western blotted using anti-GFP antibodies. For each panel, the top blot contains proteins isolated by immunoprecipitation (I.P.) and the bottom blot contains total soluble protein (total extract). (A) Full-length Rfa1 precipitates functional Rfa2- and Rfa3-GFP fusions (Rfa2-GFP, Rfa2Δ247-GFP, and Rfa3-GFP) but does not associate with nonfunctional C-terminal truncations (Rfa2Δ169-GFP and Rfa3Δ46-GFP). (B) Only full-length Rfa3-GFP was precipitated by Rfa2-PrA. (C) Rfa3-PrA binds functional Rfa1- and Rfa2-GFP fusions (Rfa1-GFP, Rfa2-GFP, and Rfa2Δ247-GFP) but not inviable truncations (Rfa1Δ353-GFP, Rfa1Δ330-GFP, and GFP).