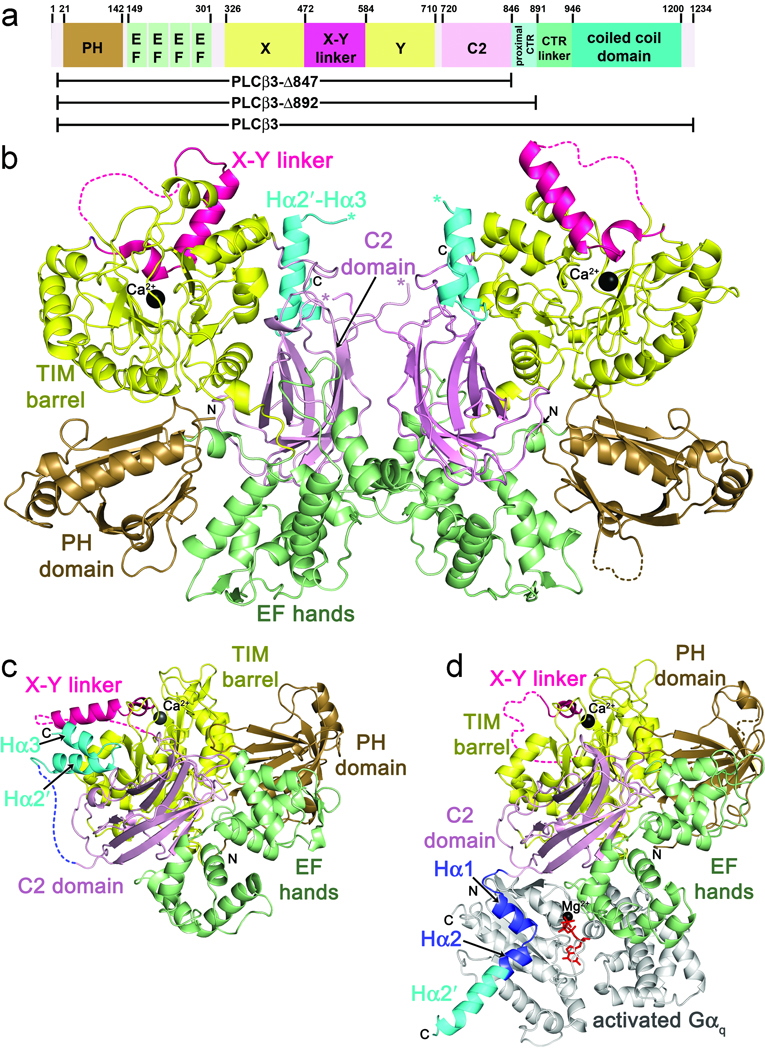
Figure 1.
Primary and tertiary structures of PLCβ family members, and comparison of cephalopod PLC21 with the Gαq–PLCβ3 complex. (a) Primary structure of human PLCβ3. PLCβ3 truncations used in this paper are indicated below the diagram. Numbers above the diagram correspond to amino acid positions at domain boundaries. (b) Crystal structure of LPLC21. LPLC21 crystallized as a dimer with pseudo two-fold symmetry. Domains are colored as in a. The Hα2′-Hα3 hairpin from the proximal CTR is shown in cyan, and the catalytic Ca2+ is shown as a black sphere. Disordered loops are drawn as dashed lines, with the exception of the connection between the C2 domain and the beginning of Hα2′, which is ambiguous in the dimer interface. The C-terminus of the C2 domain and start of Hα2′ are marked with pink and blue asterisks, respectively. N- and C-terminal ends of the protein fragment resolved in the crystal structure are labeled N and C, respectively. (c) Crystal structure of SPLC21. Domains are colored as in b. (d) Crystal structure of the Gαq–PLCβ3 complex (PDB entry 3OHM)23. Hα1 and Hα2, which form the primary Gαq binding site, are shown in dark blue. Residues corresponding to Hα2′ in the PLC21 structures are shown in cyan. Activated Gαq is shown in light gray, with GDP and AlF4 colored red, and Mg2+ black.