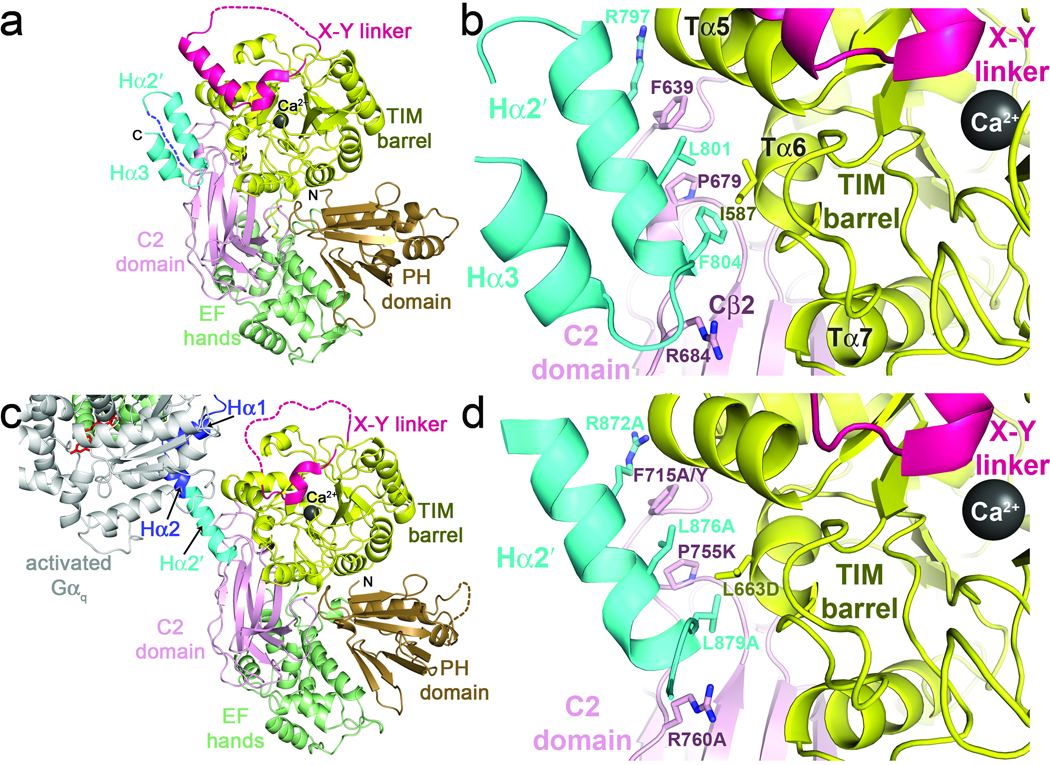
Figure 2.
Interactions of Hα2′ with the catalytic core. (a) The SPLC21 Hα2′ helix docks in a conserved cleft formed between the TIM barrel and C2 domains, in close proximity to the active site and the X–Y linker. The shorter Hα3 helix forms a hairpin interaction with Hα2′ stabilized by hydrophobic interactions. Domains are colored as in 1a. (b) Specific interactions of SPLC21 Hα2′ with the catalytic core. Side chains that make large contributions to the binding interface are shown as sticks with carbons colored according to their respective domains and nitrogens blue. (c) The Hα2′-catalytic core interaction is recapitulated in a crystal contact of the Gαq–PLCβ3 structure (PDB entry 3OHM)23. Domains are colored as in 1d. The subunit of Gαq shown is in complex with a different catalytic core in the crystal lattice. (d) Specific interactions between Hα2′ and the catalytic core in human PLCβ3 (Fig. 1c). Residues analogous to those of SPLC21 shown in b are drawn as sticks, and site-directed mutations created in this study to perturb the interface are indicated. The SPLC21 Hα2′ helix is continuous, whereas Hα2′ in human PLCβ3 is kinked at Ala877, as if to optimize the interactions of the Leu879 side chain, which is smaller than that of the corresponding Phe804 residue in SPLC21.