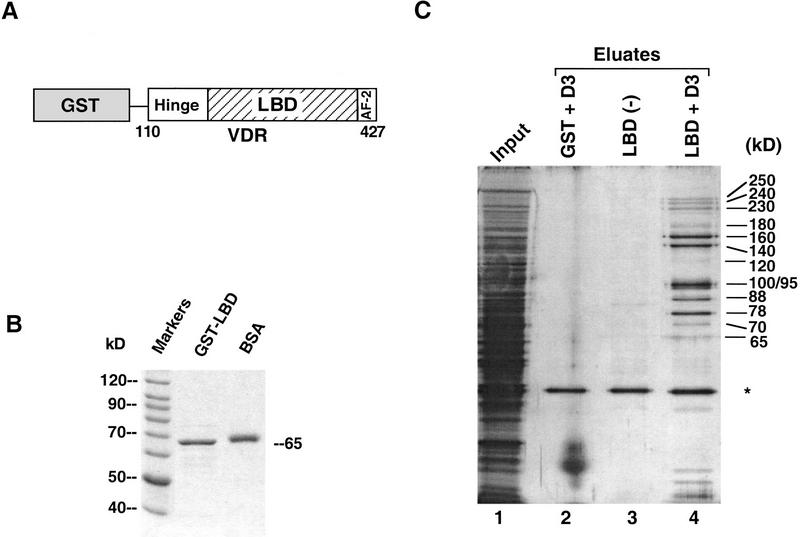
Figure 1.
The VDR ligand-binding domain interacts with several nuclear proteins in a hormone-dependent manner. (A) Schematic representation of the human VDR LBD fused to GST. The carboxyl terminus of VDR (110–427), was fused to GST. The VDR LBD includes, in addition to the ligand-binding domain, a ligand-dependent transactivation motif AF-2, and a region between the LBD and the DNA-binding domain that has been called the “hinge” to which corepressors N-CoR/SMRT interact with TR and RAR. The numbers correspond to the human VDR amino acid sequence (Baker et al. 1988). (B) Overexpression and purification of GST–VDR LBD. The bacterially overexpressed GST–VDR LBD was purified on glutathione-Sepharose beads and used as an immobilized bait [see (C)]. Five microliters of bead slurry and 0.5 μg of BSA used as a size reference are shown following separation on SDS-PAGE and detected by Coomassie blue staining.(C) Ligand-dependent interactions between VDR LBD and a number of proteins from Namalwa B-cell nuclear extracts. Immobilized GST–VDR LBD was incubated with a Namalwa nuclear extract (input, lane 1) in the absence (ethanol, lane 3) or presence of 1 μm 1,25(OH)2D3 (lane 4). VDR interacting proteins (DRIPs) were eluted from the GST–VDR LBD column by incubation with N-lauroyl sarkosine (Sarkosyl). The eluates were separated by SDS-PAGE and analyzed by silver nitrate staining. Immobilized GST (lane 2) was used as a control protein in the presence of ligand. The approximate, apparent molecular masses of each interacting protein is shown at right. The asterisk denotes a nonspecific binding protein.