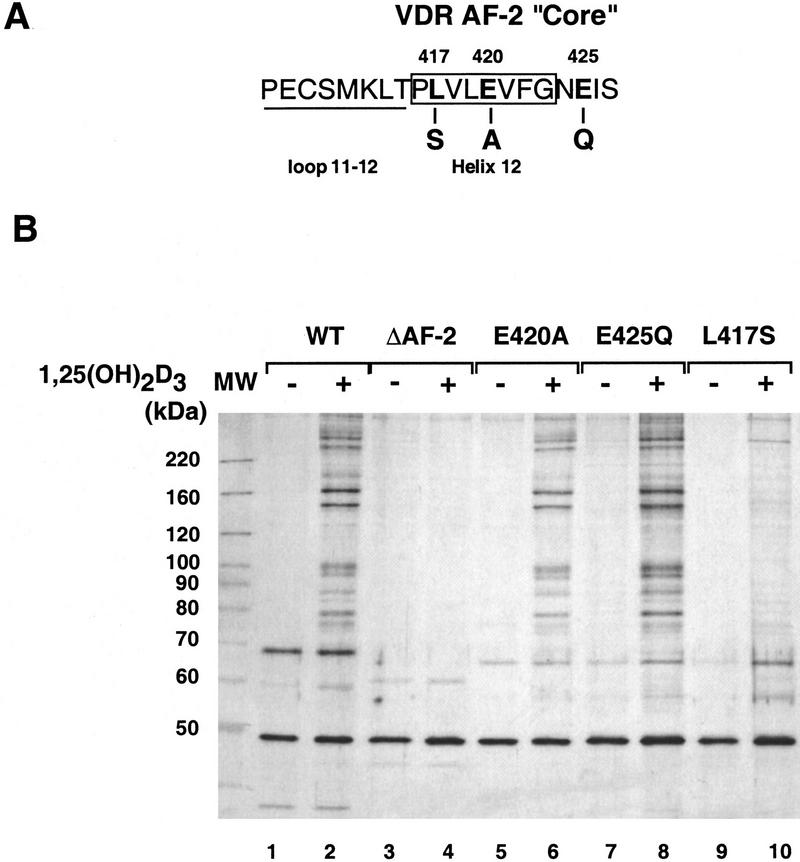
Figure 3.
DRIP interactions with VDR are AF-2-dependent. (A) Amino acid sequence of the extreme carboxyl terminus of VDR. Based on the crystal structures of three related nuclear receptor LBDs, the final loop and α-helix (helix 12), overlapping the AF-2 core, are denoted. (B) DRIP interactions with AF-2 mutants. Namalwa nuclear extracts were incubated, as described in Fig. 1, with immobilized GST fusions to the VDR LBD (WT; lanes 1,2); VDR-LBDΔAF-2 (deletion of residues 404–427, lanes 3,4); VDR LBD/E420A (lanes 5,6); VDR LBD/E425Q (lanes 7,8); VDR LBD/L417S (lanes 9,10). Each pair is shown in the absence (−) or presence (+) of 10−6 m 1,25(OH)2D3.