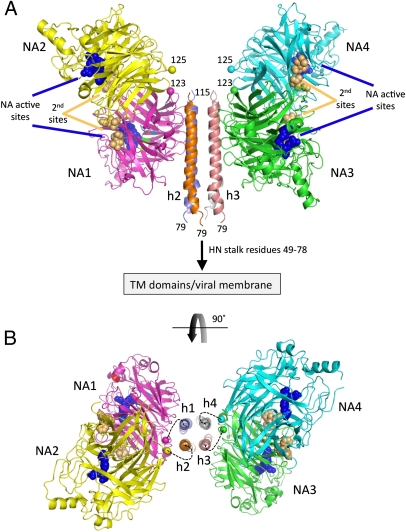
Fig. 2.
Structure of the NDV HN (AV) ectodomaim. (A) Two dimers of the NDV HN NA domains flank the 4HB in the stalk. The four NA domains are labeled NA1–NA4. The active sites are marked by three residues shown as blue CPK spheres (E400, R415, and Y525) and labeled accordingly. The secondary sialic acid binding sites located at the NA domain dimer interfaces are marked by residues shown as orange CPK spheres and labeled (second sites). The N termini of the four NA domains, residues 123 and 125, are labeled and indicated by their CA atoms shown in CPK format colored by chain. The connections of the N-terminal region of the stalk to the HN TM domains and viral membrane are indicated. (B) End-on view of the packing of the HN stalk tetramer between two NA domain dimers rotated through 90° as indicated by the curved arrow. Although no electron density was observed to connect the HN stalk helices with the individual NA domains, the dotted lines indicate possible linkages between these domains, with NA1/NA2 and NA3/NA4 forming covalently linked dimers through C123 and C92 in the S92C mutant. The four-stalk helices are indicated as h1–h4.