Abstract
The renin-angiotensin (Ang) system regulates multiple physiological functions through Ang II type 1 and type 2 receptors. Prior studies suggest an intracellular pool of Ang II that may be released in an autocrine manner upon stretch to activate surface membrane Ang receptors. Alternatively, an intracellular renin-Ang system has been proposed, with a primary focus on nuclear Ang receptors. A mitochondrial Ang system has not been previously described. Here we report that functional Ang II type 2 receptors are present on mitochondrial inner membranes and are colocalized with endogenous Ang. We demonstrate that activation of the mitochondrial Ang system is coupled to mitochondrial nitric oxide production and can modulate respiration. In addition, we present evidence of age-related changes in mitochondrial Ang receptor expression, i.e., increased mitochondrial Ang II type 1 receptor and decreased type 2 receptor density that is reversed by chronic treatment with the Ang II type 1 receptor blocker losartan. The presence of a functional Ang system in human mitochondria provides a foundation for understanding the interaction between mitochondria and chronic disease states and reveals potential therapeutic targets for optimizing mitochondrial function and decreasing chronic disease burden with aging.
Keywords: frailty, ATP, bioenergetics
The renin-angiotensin (Ang) system (RAS) is a key regulator of cardiovascular and renal function. Although many studies have focused on the impact of extracellular Ang II and its receptors, type 1 (AT1R) and type 2 (AT2R), on the cardiovascular system, others have reported that Ang II is also present in the intracellular compartment and can be released upon cell stretch to mediate cellular growth and/or apoptosis (1–3). Although many of the autocrine effects of this endogenous Ang store are believed to be mediated by plasma membrane Ang receptors, an intracellular RAS acting on nuclear Ang receptors has also been proposed (4, 5).
The RAS influences cardiovascular function via nitric oxide (NO) regulation (6–8). AT1R blockade increases NO, and this increase is abolished by concomitant AT2R blockade, suggesting that AT2Rs are important in NO production (9). AT2Rs likely increase NO production via direct stimulation of NO synthase (NOS) (10) or indirectly through bradykinin-dependent mechanisms (7). Recently, the intracrine activation of AT2Rs has been reported to increase NO production in isolated cortical kidney nuclei (11).
Possible sources of NO coupled to Ang signaling include the three canonical NOS isoforms: neuronal (nNOS), inducible (iNOS), and endothelial (eNOS) NOS (12). Additionally, there have been reports of a NOS isoform in mitochondria (mtNOS) (13, 14). Although the unique identity of mtNOS is still controversial (15), this mitochondria-specific isoform is localized to the inner mitochondrial membrane, where it may regulate mitochondrial respiration (13, 16, 17). mtNOS activity is thought to decline with aging (18). Although several studies have provided evidence that Ang receptors can couple to the canonical NOS isoforms (7), nothing is known about whether intracellular Ang II influences mitochondrial NO production or if it has any other effects on mitochondrial function. Given the known relationship between the RAS and cellular NO production, as well as the emerging understanding of intracellular RAS functionality, we hypothesized that the RAS plays an important role in intramitochondrial NO production.
Results
Several approaches were used to test our hypothesis on the presence and function of a mitochondrial Ang system (MAS). First, using high-resolution transmission immunoelectron microscopy and well-characterized antibodies against AT1R, AT2R, and Ang, we studied sections from human skeletal muscle cells and monocytes as well as mouse cardiac myocytes, renal tubular cells, neuronal cells, vascular endothelial cells, and hepatocytes. Second, using double-labeling and immunoelectron microscopy, we demonstrated colocalization of Ang receptor(s) and Ang. Third, we transfected human fibroblasts with a pcDNA-Cycle 3 GFP-AT2R construct, leading to expression of Cycle 3 GFP-AT2R, which we tracked by using confocal imaging in living cells. Fourth, we purified mitochondrial subfractions down to the inner mitochondrial membrane to determine the precise location of the Ang receptors within the mitochondria. Fifth, we measured changes in mitochondrial NO production and respiration in response to activation or inhibition of the receptor(s) in isolated mitochondria. Finally, we characterized the effects of aging and long-term blockade of AT1Rs on the MAS.
Subcellular Distribution of AT1Rs and AT2Rs.
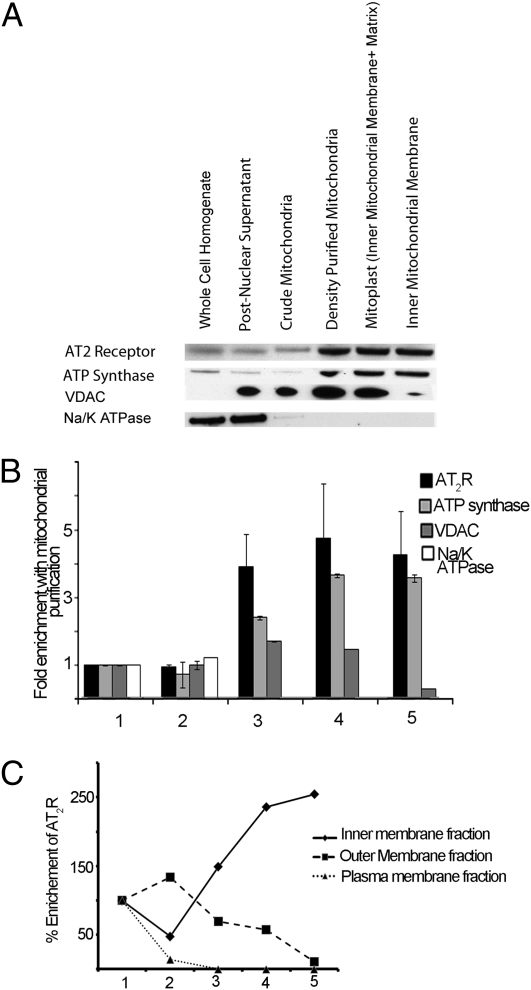
The subcellular distribution of AT1Rs and AT2Rs was assessed by high resolution immunoelectron microscopy in human monocytes, skeletal myocytes and in cardiac myocytes, renal tubular cells, neuronal cells, vascular endothelial cells, and hepatocytes from C57BL/6 mouse, using specific polyclonal antipeptide antibodies. AT1R immunoreactivity was observed in the cell membrane of human monocytes (Fig. 1A). AT1R did not appear in the mitochondria from young adult monocytes or animal tubular kidney cells (Fig. 1 B and C) except in rare occurrences (Fig. 1D), but was consistently found not far from the mitochondrial outer membrane (Fig. 1C). AT2R immunoreactivity was observed in the cell membrane of human monocytes (Fig. 2A). Abundant mitochondrial AT2R (mtAT2R) was also observed in human monocytes and mouse tissues (Fig. 2 B–D).To confirm the immunoelectron microscopy findings, mouse heart homogenates were fractionated first by differential centrifugation and subsequently by density gradient centrifugation. The density-purified mitochondria were probed with specific antibodies. AT2R immunoreactivity increased with progressive purification of mitochondria, for which cytochrome c oxidase (CoxIV) was used as a marker. Importantly, the immunoreactivity of Na+/K+ ATPase, a plasma membrane marker, declined with progressive enrichment of mitochondria. This is consistent with mitochondrial enrichment of AT2Rs (Fig. S1 A and B). In contrast, AT1Rs did not enrich with mitochondrial fractions.
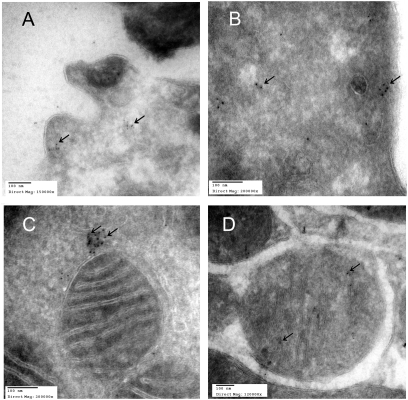
Fig. 1.
Immuno-electron microscopic localization of AT1Rs in sections of human monocyte (A and B) and mouse kidney tubular cells (C and D) using gold bead labeling (arrows) for AT1Rs. A shows AT1Rs on human monocyte cell membrane and in the cytoplasm (B). C shows labeling in close proximity to mitochondria and rarely in the mitochondria (D).
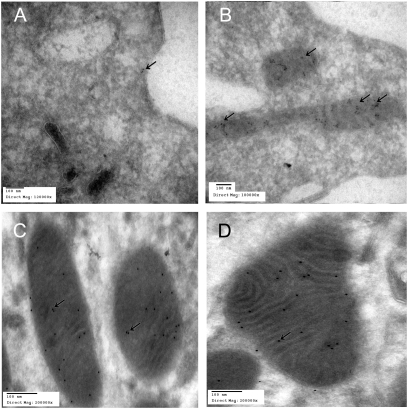
Fig. 2.
Immuno-electron microscopic localization of AT2Rs in sections of human monocyte (A and B) and mouse renal tubular cells (C and D) using gold beads labeling (arrows) for AT2Rs. A shows AT2Rs on human monocyte cell membrane, and B–D reveal heavy labeling for AT2Rs within mitochondria.
We quantified through EM the relative expression of AT2Rs per mitochondria in cells obtained from different human and mouse tissues (Fig. S1C).
Colocalization of Ang and AT2Rs.
Given that a functional MAS would require the presence of local Ang to activate the receptor, we next sought evidence for mitochondrial Ang by immunoelectron microscopy. Using a gold-conjugated secondary antibody to anti-Ang antibody (6 nm gold), we demonstrated the presence of mitochondrial Ang in mouse hepatocytes (Fig. 3A), kidney tubular cells (Fig. 3B), brain neurons (Fig. 3C), and heart myocytes (Fig. 3D). Moreover, Ang was not randomly distributed within mitochondria; rather, it colocalized with mtAT2R, detected with a gold-conjugated secondary antibody to anti-AT2R antibody (12 nm gold). The abundant distribution of mtAT2R in the young animals, observed in electron microscope images, was correlated with a scarcity of mtAT1R. Despite the observation of many Ang immunolabeled particles in electron microscope images, Ang was not detected in Western blots of isolated, density-purified mitochondria, indicating that Ang is loosely bound and is lost upon washing the isolated mitochondria.
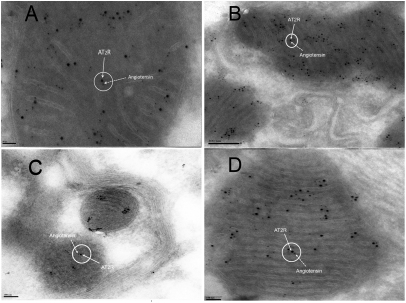
Fig. 3.
Immunoelectron microscopic localization of AT2R binding to Ang in the mitochondria by using a gold-labeled anti-AT2R antibody (12 nm gold) and a gold-labeled anti-Ang antibody (6 nm gold). Shown is colocalization of AT2Rs with Ang in sections of mouse hepatocytes (A), kidney tubular cells (B), neurons (C), and cardiac myocytes (D).
AT2R Transfection of Fibroblast Cells.
To ensure that the results described above were not attributable to nonspecific or off-target binding of antibody to mitochondrial targets, we confirmed mitochondrial localization of AT2Rs by using a GFP-AT2R fusion construct. Briefly, human fibroblasts were transfected with pcDNA-Cycle 3 GFP-AT2R or positive control using pcDNA-EGFP-C1 before counterstaining with the mitochondrial fluorescence marker MitoTracker Red. GFP-AT2R fluorescence was confined to discrete intracellular puncta (Fig. 4B), mirroring the distribution of MitoTracker Red (Fig. 4A). Spatial overlap of the two signals, denoting subcellular colocalization, is shown in Fig. 4C. The extent of colocalization was assessed quantitatively over a series of confocal Z sections (0.37 μm); spatial signal correlation between the two fluorophores was high (R2 = 0.72). Thus, the high density of AT2Rs observed in the mitochondria in electron microscope images was corroborated by tracking the Cycle 3 GFP-AT2R fusion protein in live human fibroblasts, where it was predominantly colocalized with the mitochondrial marker MitoTracker Red.
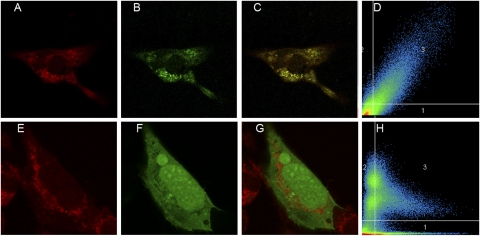
Fig. 4.
Transfected AT2Rs colocalize with mitochondria in human fibroblasts. Human fibroblast cells were transfected with pcDNA-Cycle 3 GFP-AT2R construct (B) or positive control using pcDNA-EGFP-C1 (F) and counterstained with MitoTracker Red (A and E) (100× oil immersion). The merged images show yellow fluorescence (C and G). Fluorographic analysis (D and H) reveals a high correlation coefficient (R2 = 0.72), suggesting a strong colocalization between AT2Rs and MitoTracker within the mitochondria.
AT2R Localization Within Mitochondria.
Having confirmed the presence of mtAT2Rs by two independent methods, we sought to determine the precise location of these receptors within the mitochondria. Further inspection of the immunoelectron micrographs in Figs. 2 and 3 suggested that the mtAT2R is resident in the inner, rather than the outer, mitochondrial membranes. To confirm this observation by a different method, we isolated inner mitochondrial membrane-enriched membranes from mouse liver according to previously published methods (19). We determined the specific immunoreactivity of AT2R in the inner mitochondrial membrane as described in the previous section. AT2R immunoreactivity copurified with that of ATP synthase β, an inner mitochondrial membrane marker. In contrast, markers of the plasma membrane (Na+/K+ ATPase) and the outer mitochondrial membrane [voltage-dependent anion channel (VDAC)] were progressively removed by inner mitochondrial membrane purification (Fig. 5A). These results buttress the results of the immunoelectron microscopy studies and are consistent with localization of AT2R in the inner mitochondrial membrane.
Fig. 5.
Purification of inner mitochondrial membrane AT2Rs. (A) Whole-liver homogenate fractionations up to the inner mitochondrial membrane were subjected to 12% SDS/PAGE and immunoblotting with anti-AT2R as well as anti-Na+/K+ ATPase, anti-VDAC, and anti-ATP synthase β for detecting cell membrane, outer mitochondrial membrane, and inner mitochondrial membrane markers, respectively. AT2Rs tracked with inner mitochondrial membrane marker ATP synthase β, consistent with inner mitochondrial membrane localization of AT2Rs. (B) Integrated densitometric band analysis of immunoblots demonstrating fold enrichment of AT2Rs, inner mitochondrial membrane marker (ATP synthase), outer mitochondrial membrane marker (VDAC), and plasma membrane marker (Na/K ATPase) with mitochondrial purification. (C) Percentage enrichment of AT2Rs with cellular subfractions through mitochondrial purification. Fractions: 1, whole-cell lysate; 2, postnuclear (480 × g); 3, postdifferential centrifugation (7,700 × g); 4, post-HistoDenz gradient centrifugation (50,500 × g); 5, postsucrose gradient centrifugation (77,000 × g).
AT2Rs Modulate Mitochondrial NO Production.
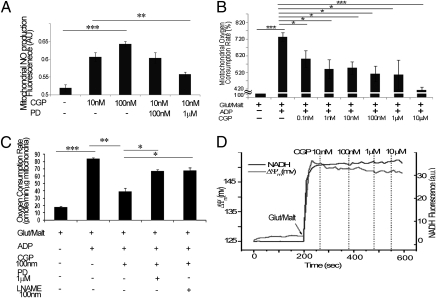
To determine whether mtAT2R activation might modulate mitochondrial NO production in a manner analogous to the actions of non-mtAT2Rs on other NO isoforms, we examined the direct effects of AT2R agonists and antagonists on isolated kidney mitochondria. Transmission electron microscopy and Western blot analyses were used to confirm minimal contamination of the isolated mitochondria with other cell fractions and a typical morphology of mitochondria. The NO fluorescent molecular detection probe kit (Enzo Life Sciences) was used according to the manufacturer’s instructions (20). Isolated mitochondria were treated for 30 min with the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO) followed by a 15-min incubation with the AT2R agonist CGP421140 and/or the antagonist PD-123319. Positive control samples were treated with the NOS substrate l-arginine, and negative control samples were generated by treatment with NO scavenger (c-PTIO). Isolated mitochondria were treated and then incubated with a specific fluorescent probe for real-time measurement of NO. Fluorescence signal was measured by using a multimode microplate reader equipped with Cyanine 5 (650/670 nm). Treatment of mitochondria with CGP421140 caused a concentration-dependent increase in mitochondrial NO production (Fig. 6A); specifically, 10 nM was sufficient to stimulate NO production [from 0.52 ± 0.0009 fluorescence arbitrary units (AU) at baseline control to 0.6 + 0.01 AU, P < 0.001]. Moreover, CGP421140-stimulated NO production was mitigated by pretreatment with PD-123319 (1 μΜ; 0.55 ± 0.006 AU, P < 0.01; Fig. 6A).
Fig. 6.
mtAT2R modulation of mitochondrial respiration and NO production. (A) Increased mitochondrial NO production in response to 10 nM and 100 nM concentrations of the AT2R agonist CGP421140 (CGP), which can be reversed with the addition of a 1 μM concentration of the AT2R antagonist PD-123319 (PD). (B) Mitochondrial respiration decreased significantly in response to serially increasing concentrations of CGP421140. Linear regression of CGP421140 concentrations versus oxygen consumption was significant at P < 0.0004. (C) Decreased respiration in response to AT2R agonist CGP421140 at 100 nM was reversed with the addition of AT2R antagonist PD-123319 at 1 μM or an inhibitor of NO production, l-NAME, at 100 nM. (D) No changes in mitochondrial membrane potential (ΔΨm) are evident in response to CGP421140 at increasing concentrations, confirming that effects observed on mitochondrial respiration are not attributable to nonspecific effects of CGP421140 on mitochondrial bioenergetic parameters. (*P < 0.05, **P < 0.005, ***P < 0.0005.)
AT2Rs Modulate Mitochondrial Respiration.
To determine how mtAT2R stimulation might influence global mitochondrial function, we examined effects on respiration in rat heart mitochondria. Glutamate/malate-supported respiration (state 2) (21) was initially 17.8 ± 1.06 pmol/min per μg, Addition of ADP increased respiration (state 3) to 84.12 ± 1.24 pmol/min per μg (P < 0.0005). We subsequently found that the addition of 100 nM CGP421140 caused a significant decrease in state 3 respiration (Fig. 6C; 39.47 ± 4.37 pmol/min per μg, P < 0.005). The dose of 100 nM CGP421140 was selected based on a series of experiments used to determine the dose of CGP421140 needed to influence mitochondrial respiration (Fig. 6B). The effects of CGP421140 were not likely attributable to nonspecific uncoupling because control experiments showed that 100 nM CGP421140 had little effect on mitochondrial membrane potential (ΔΨm) or NADH level (Fig. 6D). Importantly, CGP421140-mediated inhibition of respiration was prevented by blocking the AT2R; PD-123319 (1 μM) restored CGP421140-inhibited state 3 respiration (Fig. 6C; 66.83 ± 2.1 pmol/min per μg, P < 0.01). The addition of l-NG-nitroarginine methyl ester (l-NAME; 1 μM), an arginine analog that inhibits NO production, reversed the CGP421140 effect on mitochondrial respiration (Fig. 6C; 67.4 ± 4.3 pmol/min per μg, P < 0.05), indicating that the functional effects of mtAT2R activation on mitochondrial respiration are via an NO-dependent mechanism.
To further validate this effect of MAS on mitochondrial respiration, we studied liver mitochondria with different mitochondrial substrates. Liver mitochondria respiring with glutamate/malate as the substrate gave results similar to those obtained in isolated cardiac mitochondria. The AT2R agonist inhibited state 3 respirations in the nanomolar range for both glutamate/malate- and succinate-supported respiration. In succinate-supported respiration, the inhibitory response was slightly more pronounced at 1 nM than for glutamate/malate-supported respiration (Fig. S2).
Age-Related Changes in the MAS.
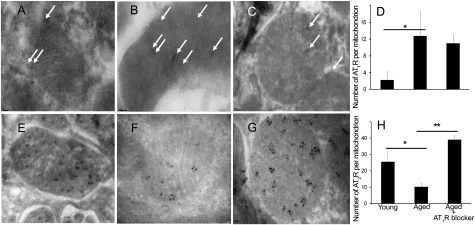
Although still controversial, substantial evidence documents a decline in the canonical Ang system with age (22–24), and mtNOS activity is reportedly reduced with aging (18). We examined the effect of aging on the MAS. Kidney tubular cell sections from 20- or 70-wk-old C57BL/6 mice and aged (70-wk-old) mice treated with losartan at a dose of 40–60 mg/kg per day for 20 wk were labeled with antibodies and visualized with immunoelectron microscopy. Representative mitochondria, labeled for AT1R (Fig. 7 A–C) and AT2R (Fig. 7 D–F) are shown. Younger mice (Fig. 7 A and D) and older mice treated with losartan (Fig. 7 C and F) had similar densities of labeled AT2R, whereas older untreated mice (Fig. 7 B and E) had a lower AT2R density. Our results demonstrate a significant decrease in the expression of mtAT2R with aging (from 25.5 + 6.2 to 10.2 + 2.2 gold-labeled AT2R per mitochondrion, P < 0.001) that is reversed by treatment with losartan (39.0 + 2.6 gold-labeled AT2R per mitochondrion). In contrast, mtAT1R was significantly increased with aging (from 2.0 + 2.2 to 12.7 + 5.8 gold-labeled AT1R per mitochondrion, P < 0.001). This increase was slightly attenuated with chronic AT1R blockade (11.0 + 2.1 gold-labeled AT1R per mitochondrion).
Fig. 7.
Effect of AT1R blocker losartan on expression of mitochondrial AT1Rs and AT2Rs. Renal tubular cell sections from C57BL/6 20 wks old (A and E), 70 wks old (B and F), and 70 wks old treated with losartan 40-60 mg/kg/day for 20 wks (C and G). D and H represent average counts of immune-labeling densities of mtAT1R (D) and mtAT2R (H) by age group in response to losartan. Gold particles in 30 mitochondria from each immunolabeling experiment were counted and averaged. Age was associated with a significant decrease in mtAT2R that was reversed with chronic use of losartan. *P < 0.005, **P < 0.0005.
Discussion
Virtually every organ system in the human body has been shown to possess a local Ang signaling system. The components of the local Ang systems are present in peripheral tissues such as the vasculature, kidneys, adrenal glands, liver, heart, and immune cells, all of which locally produce Ang II. An intracellular RAS has also been proposed, whereby Ang II produced by intracellular angiotensinogen acts on nuclear Ang receptors (11, 25). The present findings, which document the subcellular localization of functional MAS coupled to AT2R, open an area of investigation into the regulation of mitochondrial function by Ang II-mediated intracrine signaling. Although previous reports do not specifically anticipate the localization of Ang receptors on mitochondria, some clues may be gleaned from prior work (5, 26–29). Early studies indicated that radio-labeled Ang II could be associated with the mitochondria (30), and chronic exposure to Ang II had a significant stimulatory effect on rat mitochondrial growth and proliferation (31) as well as improved coupling of respiration and ATP synthesis in intact mitochondria (27).
How these receptors are transported to the mitochondria is still unclear. Many mitochondrial proteins are synthesized as cytosolic precursors, containing N-terminal mitochondrial localization sequences. Cytosolic chaperones then deliver the precursor proteins to the transport machinery of the mitochondrial outer and inner membranes. Neither AT1Rs nor AT2Rs possess a consensus targeting sequence. Nevertheless, a number of other mitochondrial transmembrane proteins [e.g., mitochondrial carrier proteins such as adenine nucleotide translocase (32)] also do not contain a predictable mitochondrial signal sequence (33), and the absence of this criterion does not exclude trafficking to the mitochondria.
The localization of AT2Rs in the mitochondrial inner membrane suggests their importance to NO production, which is believed to originate in the inner membrane through mtNOS (16, 34, 35). NO production was dose-dependently activated by the AT2R agonist CGP421140 in isolated mitochondria, and this effect was antagonized by AT2R blocker PD-123319. The observation that there was no change in ΔΨm or NADH and that mitochondrial respiration remained well-coupled at much higher doses of CGP421140 makes it unlikely that the observed responses were attributable to nonspecific toxic effects of CGP421140 on mitochondrial energetics. Moreover, because the NO response occurred in isolated mitochondria and the AT2R was localized to the inner mitochondrial membrane, the data could be taken as support for a mitochondrially localized NOS, a currently controversial subject. Whether AT2R-coupled NO is produced on the matrix side of the inner membrane remains to be determined.
Mitochondrial NO production has been reported to reversibly inhibit mitochondrial respiration by binding to CoxIV (36). We observed inhibition of respiration by the AT2R agonist in parallel with NO production. Importantly, CGP421140-reduced respiration was reversed by the addition of the NO blocker l-NAME, indicating that the functional effects of mtAT2R activation on mitochondrial respiration is via an NO-dependent mechanism. Alternatively, downstream effects of NO, such as activation of cGMP production and posttranslational modification of mitochondrial targets, could also account for the observed decrease in oxygen consumption. Further investigation will be necessary to determine whether the AT2R effect on respiration depends on NO or cGMP.
The decrease in mtAT2R and increase in mtAT1R density with aging, and the reversal of these changes with long-term AT1R blockade, fits with the general model of antagonism between AT1Rs and AT2Rs (37) as well as with reports that AT1R blockers up-regulate AT2R expression and function (38). In addition, AT1R blockers have been reported to reduce age-related mitochondrial dysfunction, attenuate hypertension-induced renal mitochondrial dysfunction, and protect against cardiac mitochondrial dysfunction in the setting of acute ischemia (39, 40). Interestingly, disruption of AT1Rs was associated with an increased number of mitochondria and up-regulation of the prosurvival genes nicotinamide phosphoribosyltransferase (Nampt) and sirtuin 3 (Sirt3) in the kidney, leading to marked prolongation of life span in mice (41).
Although Ang II is the major effector peptide of the RAS, recent evidence suggests that its metabolite des-aspartyl1-Ang II (Ang III) plays an important role in the activation of AT2Rs (42). Ang III is more membrane-permeable than Ang II and the most prevalent Ang peptide in the brain, and perhaps inside the cell, because of its lipophilicity (43, 44). Because of the minimal structural difference between Ang II and Ang III, it is difficult to distinguish between the two peptides via most Ang II-specific antibodies. Given this fact and the evidence of the important role that Ang III plays in activation of the Ang system (44), the effects seen in the MAS may perhaps be driven by Ang III rather than Ang II. Hence, the role that the active metabolites of Ang II play in the activation of the MAS will require further study.
A recent study reported that AT2Rs can act as either an antioxidative or a prooxidative receptor (45), depending on the conditions. Further studies are needed to determine the role of the MAS in the production of ROS and its potential effects in the mitochondria and cellular environment.
In the light of our present study and recent reports on the effects of AT1R blockers on mitochondrial number and function, such effects might be mediated via unopposed mtAT2Rs. Given the established role of NO in cardioprotection (46), it is tempting to speculate that the MAS could be involved in mitigation of ischemic or age-related mitochondrial injury. Although many more studies are required to define the role that this system plays in the function of mitochondria in each organ system, the identification and characterization of a functional MAS opens up avenues of research involving the Ang system.
Methods
The following sections contain a brief overview of the methods used for results presented in this article. For further details of these methodologies, please see SI Methods.
Isolation of Mitochondria.
For functional assays, crude mitochondria from animal groups were separated by using differential centrifugation (47). For structural studies, mouse liver, heart, kidney, and brain cells were subjected to fractionation (19).
Western Blot Analysis.
Mitochondrial preparations were resolved by 12% SDS/PAGE, transferred to the nitrocellulose membranes, and probed against Na+/K+ ATPase, CoxIV, VDAC, ATP synthase β, AT2R, Ang, and AT1R.
Transfection of Human Fibroblast Cells.
Human fibroblast cells were transiently transfected with pcDNA-Cycle 3 GFP-AT2R, positive control pcDNA-EGFP, or negative control pcDNA AT2R-Cycle 3 GFP. Images were acquired with the Zeiss Meta Confocal Microscope System.
Mitochondrial NO Production.
Isolated mitochondria were incubated with AT2R agonists (CGP421140 at 10 nM and 100 nM) and antagonists (PD-123319 at 100 nM and 1 μM). The NO fluorescent molecular detection probe kit (Enzo Life Sciences) was used according to the manufacturer’s instructions (20).
Immunolocalization of AT1R, AT2R, and Ang II.
Immunogold electron microscopy was performed as described (48). The sections were probed against AT2R, Ang, or AT1R antibodies.
Mitochondrial Respiration.
Isolated rat heart or liver mitochondria were aliquoted into a XF96 microplate (Seahorse Bioscience) (49). AT2R agonist and antagonist and an arginine analog that inhibits NO production (l-NAME) were added individually or combined, and oxygen consumption rates were determined by using a compartment model-based algorithm.
Mitochondrial Membrane Potential Measurements.
Changes in isolated mitochondria membrane potential (ΔΨm) were quantified from changes in tetramethylrhodamine methyl ester (TMRM)-positive fluorescence in response to increasing concentrations of AT2R agonist CGP421140 being added to the mixture (50).
Human and Animal Groups.
Mitochondria were derived from young adult humans (20–30 y old), adult rats (24 wk old), adult (20 wk old) and aged (70 wk old) mice, and a human cell line. Adult mice at 50 wk of age were treated with the AT1R blocker losartan at doses of 40–60 mg/kg per day for 20 wk.
Statistical Analyses.
Data are expressed as means ± SD and analyzed with the one-way ANOVA program. Differences were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
This study was supported by the Johns Hopkins Older Americans Independence Center National Institute on Aging Grant P30 AG021334, T. Franklin Williams Scholars Award of the American Geriatrics Society, National Institute on Aging Grant K23 AG035005-01, John A. Hartford Center of Excellence Award, Johns Hopkins Clinical Scientist Awards (to P.M.A.), and National Institutes of Health Grant R01-HL101235 (to B.O.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1101507108/-/DCSupplemental.
References
- 1.Leri A, et al. Stretch-mediated release of angiotensin II induces myocyte apoptosis by activating p53 that enhances the local renin-angiotensin system and decreases the Bcl-2-to-Bax protein ratio in the cell. J Clin Invest. 1998;101:1326–1342. doi: 10.1172/JCI316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leri A, et al. Up-regulation of AT1 and AT2 receptors in postinfarcted hypertrophied myocytes and stretch-mediated apoptotic cell death. Am J Pathol. 2000;156:1663–1672. doi: 10.1016/S0002-9440(10)65037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell. 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 4.Cook JL, Zhang Z, Re RN. In vitro evidence for an intracellular site of angiotensin action. Circ Res. 2001;89:1138–1146. doi: 10.1161/hh2401.101270. [DOI] [PubMed] [Google Scholar]
- 5.Robertson AL, Jr, Khairallah PA. Angiotensin II: Rapid localization in nuclei of smooth and cardiac muscle. Science. 1971;172:1138–1139. doi: 10.1126/science.172.3988.1138. [DOI] [PubMed] [Google Scholar]
- 6.Wiemer G, Schölkens BA, Wagner A, Heitsch H, Linz W. The possible role of angiotensin II subtype AT2 receptors in endothelial cells and isolated ischemic rat hearts. J Hypertens Suppl. 1993;11:S234–S235. [PubMed] [Google Scholar]
- 7.Ratliff BB, Sekulic M, Rodebaugh J, Solhaug MJ. Angiotensin II regulates NOS expression in afferent arterioles of the developing porcine kidney. Pediatr Res. 2010;68:29–34. doi: 10.1203/PDR.0b013e3181e12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Natl Acad Sci USA. 1999;96:6506–6510. doi: 10.1073/pnas.96.11.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siragy HM, de Gasparo M, Carey RM. Angiotensin type 2 receptor mediates valsartan-induced hypotension in conscious rats. Hypertension. 2000;35:1074–1077. doi: 10.1161/01.hyp.35.5.1074. [DOI] [PubMed] [Google Scholar]
- 10.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 11.Gwathmey TM, et al. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484–F1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michel T, Feron O. Nitric oxide synthases: Which, where, how, and why? J Clin Invest. 1997;100:2146–2152. doi: 10.1172/JCI119750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates TE, Loesch A, Burnstock G, Clark JB. Immunocytochemical evidence for a mitochondrially located nitric oxide synthase in brain and liver. Biochem Biophys Res Commun. 1995;213:896–900. doi: 10.1006/bbrc.1995.2213. [DOI] [PubMed] [Google Scholar]
- 14.Valdez LB, Boveris A. Mitochondrial nitric oxide synthase, a voltage-dependent enzyme, is responsible for nitric oxide diffusion to cytosol. Front Biosci. 2007;12:1210–1219. doi: 10.2741/2139. [DOI] [PubMed] [Google Scholar]
- 15.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:579–599. doi: 10.1089/ars.2007.1845. [DOI] [PubMed] [Google Scholar]
- 16.Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–296. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- 17.Carreras MC, et al. Modulation of liver mitochondrial NOS is implicated in thyroid-dependent regulation of O2 uptake. Am J Physiol Heart Circ Physiol. 2001;281:H2282–H2288. doi: 10.1152/ajpheart.2001.281.6.H2282. [DOI] [PubMed] [Google Scholar]
- 18.Valdez LB, et al. Heart mitochondrial nitric oxide synthase. Effects of hypoxia and aging. Mol Aspects Med. 2004;25:49–59. doi: 10.1016/j.mam.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 19.Maisterrena B, Comte J, Gautheron DC. Purification of pig heart mitochondrial membranes. Enzymatic and morphological characterization as compared to microsomes. Biochim Biophys Acta. 1974;367:115–126. doi: 10.1016/0005-2736(74)90036-4. [DOI] [PubMed] [Google Scholar]
- 20.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Radic Biol Med. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 21.Nicholls DG, Ferguson SJ. Bioenergetics 3. 3rd Ed. London: Academic; 2002. [Google Scholar]
- 22.Anderson S. Ageing and the renin-angiotensin system. Nephrol Dial Transplant. 1997;12:1093–1094. doi: 10.1093/ndt/12.6.1093. [DOI] [PubMed] [Google Scholar]
- 23.Reckelhoff JF, Baylis C. Proximal tubular metalloprotease activity is decreased in the senescent rat kidney. Life Sci. 1992;50:959–963. doi: 10.1016/0024-3205(92)90174-n. [DOI] [PubMed] [Google Scholar]
- 24.Thompson MM, Oyama TT, Kelly FJ, Kennefick TM, Anderson S. Activity and responsiveness of the renin-angiotensin system in the aging rat. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1787–R1794. doi: 10.1152/ajpregu.2000.279.5.R1787. [DOI] [PubMed] [Google Scholar]
- 25.Haller H, Lindschau C, Erdmann B, Quass P, Luft FC. Effects of intracellular angiotensin II in vascular smooth muscle cells. Circ Res. 1996;79:765–772. doi: 10.1161/01.res.79.4.765. [DOI] [PubMed] [Google Scholar]
- 26.Kent KM, Goodfriend TL, McCallum ZT, Dempsey PJ, Cooper T. Inotropic agents in hypoxic cat myocardium: Depression and potentiation. Circ Res. 1972;30:196–204. doi: 10.1161/01.res.30.2.196. [DOI] [PubMed] [Google Scholar]
- 27.Allmann D, Page IH. Angiotensin. Berlin: Springer; 1974. [Google Scholar]
- 28.Goodfriend TL, et al. Clinical and conceptual uses of angiotensin receptors. In: Genest J, Koiw E, editors. Hypertension 1972. New York: Springer; 1972. pp. 549–563. [Google Scholar]
- 29.Goodfriend T, Fyhrquist F, Allmann D. Biochemical effects of angiotensins. In: Page I, Bumpus FM, editors. Handbook of Experimental Pharmacology. New York: Springer; 1974. pp. 511–517. [Google Scholar]
- 30.Glossmann H, Baukal A, Catt KJ. Angiotensin II receptors in bovine adrenal cortex. Modification of angiotensin II binding by guanyl nucleotides. J Biol Chem. 1974;249:664–666. [PubMed] [Google Scholar]
- 31.Mazzocchi G, Robba C, Rebuffat P, Nussdorfer GG. Investigations on the turnover of adrenocortical mitochondria. XV. A stereological study of the effect of chronic treatment with angiotensin II on the size and number of the mitochondria in the zona glomerulosa of the rat. Cell Tissue Res. 1980;210:333–337. doi: 10.1007/BF00237620. [DOI] [PubMed] [Google Scholar]
- 32.Pfanner N, Hoeben P, Tropschug M, Neupert W. The carboxyl-terminal two-thirds of the ADP/ATP carrier polypeptide contains sufficient information to direct translocation into mitochondria. J Biol Chem. 1987;262:14851–14854. [PubMed] [Google Scholar]
- 33.Jensen RE, Dunn CD. Protein import into and across the mitochondrial inner membrane: Role of the TIM23 and TIM22 translocons. Biochim Biophys Acta. 2002;1592:25–34. doi: 10.1016/s0167-4889(02)00261-6. [DOI] [PubMed] [Google Scholar]
- 34.Dedkova EN, Blatter LA. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J Physiol. 2009;587:851–872. doi: 10.1113/jphysiol.2008.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacza Z, et al. Mitochondrial NO and reactive nitrogen species production: Does mtNOS exist? Nitric Oxide. 2006;14:162–168. doi: 10.1016/j.niox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 37.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 38.Guan H, et al. Nitric oxide and the depressor response to angiotensin blockade in hypertension. Hypertension. 1996;27:19–24. doi: 10.1161/01.hyp.27.1.19. [DOI] [PubMed] [Google Scholar]
- 39.de Cavanagh EM, et al. Renal mitochondrial dysfunction in spontaneously hypertensive rats is attenuated by losartan but not by amlodipine. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1616–R1625. doi: 10.1152/ajpregu.00615.2005. [DOI] [PubMed] [Google Scholar]
- 40.Monteiro P, Duarte AI, Gonçalves LM, Providência LA. Valsartan improves mitochondrial function in hearts submitted to acute ischemia. Eur J Pharmacol. 2005;518:158–164. doi: 10.1016/j.ejphar.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Benigni A, et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Padia SH, et al. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51:460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 43.Chua HL, Jois S, Sim MK, Go ML. Transport of angiotensin peptides across the Caco-2 monolayer. Peptides. 2004;25:1327–1338. doi: 10.1016/j.peptides.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Reaux A, Fournie-Zaluski MC, Llorens-Cortes C. Angiotensin III: A central regulator of vasopressin release and blood pressure. Trends Endocrinol Metab. 2001;12:157–162. doi: 10.1016/s1043-2760(01)00381-2. [DOI] [PubMed] [Google Scholar]
- 45.Sabuhi R, Ali Q, Asghar M, Al-Zamily NR, Hussain T. Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: Opposing effects in lean and obese Zucker rats. Am J Physiol Renal Physiol. 2011;300:F700–F706. doi: 10.1152/ajprenal.00616.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Cavadini P, Gakh O, Isaya G. Protein import and processing reconstituted with isolated rat liver mitochondria and recombinant mitochondrial processing peptidase. Methods. 2002;26:298–306. doi: 10.1016/S1046-2023(02)00035-X. [DOI] [PubMed] [Google Scholar]
- 48.Chen JQ, Delannoy M, Cooke C, Yager JD. Mitochondrial localization of ERα and ERβ in human MCF7 cells. Am J Physiol Endocrinol Metab. 2004;286:E1011–E1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- 49.Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: Spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scaduto RC, Jr, Grotyohann LW. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys J. 1999;76:469–477. doi: 10.1016/S0006-3495(99)77214-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.