Table 2.
Cycloisomerization reaction of nitrogen- and oxygen-linked 1,6-enynes.
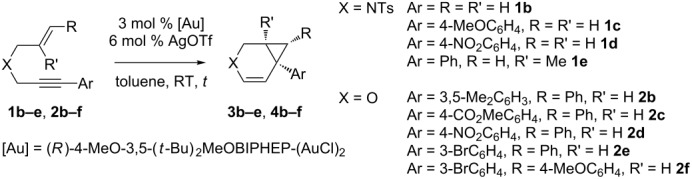 | |||||||
| Entry | Enyne | t [h] | Yield [%]a | Product | ee [%]b | ||
| 1c | 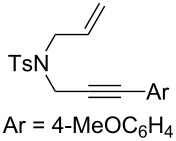 |
1c | 17 | 8 | 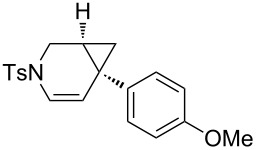 |
3c | 77 |
| 2c,d | 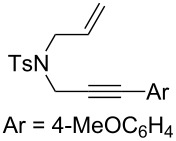 |
1c | 17 | 8 | 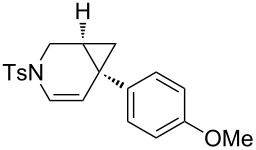 |
3c | 89 |
| 3c | 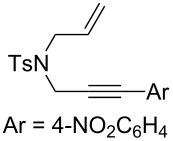 |
1d | 24 | 7 | 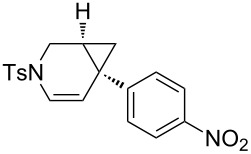 |
3d | 35 |
| 4c | 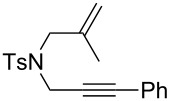 |
1e | 16 | 61 | 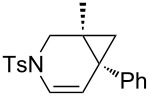 |
3e | 13 |
| 5c | 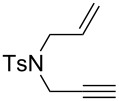 |
1b | 16 | 23 | 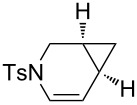 |
3b | 22 |
| 6 | 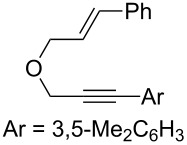 |
2b | 3.5 | 54 | 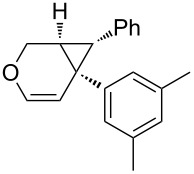 |
4b | 93 (+) |
| 7 | 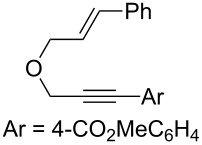 |
2c | 15 | 25 | 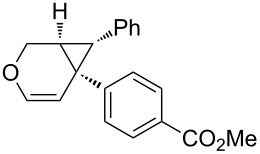 |
4c | 94 (−) |
| 8d | 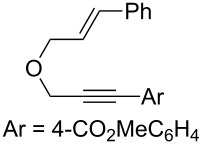 |
2c | 15 | 64 | 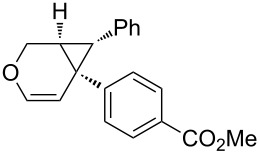 |
4c | 94 (−) |
| 9 | 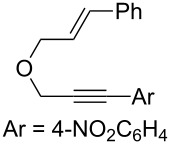 |
2d | 15 | 32 | 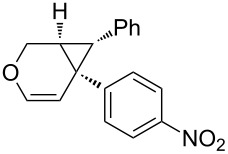 |
4d | 96 (−) |
| 10d | 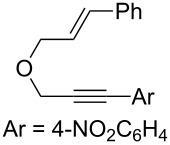 |
2d | 15 | 63 | 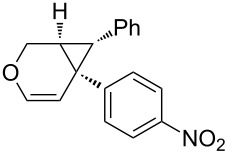 |
4d | 98 (−) |
| 11 | 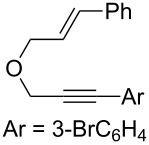 |
2e | 30 | 59 | 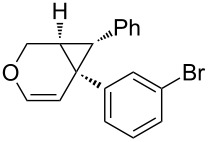 |
4e | 95 (−) |
| 12 | 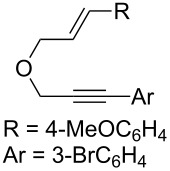 |
2f | 1 | 37 | 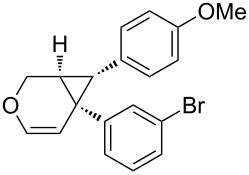 |
4f | 95 (−) |
aIsolated yield, bdetermined by HPLC, c60 °C, dAgNTf2.
