Figure 2.
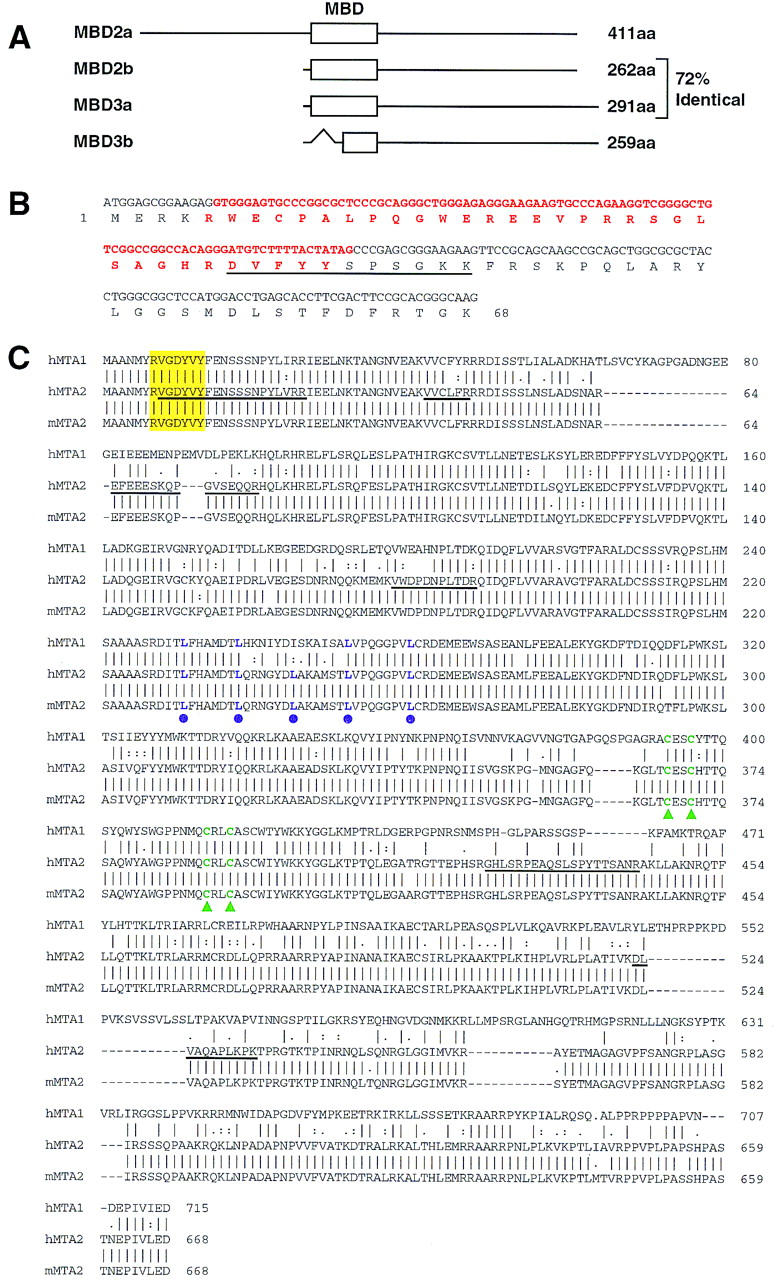
Sequence analysis of MBD3 and MTA2. (A) Schematic representation of the two forms of MBD2 and MBD3. The open box represent the methyl-CpG binding domain (MBD) initially identified in MeCP2 (also see B). The GenBank accession nos. for MBD2 and MBD3 are AF072242 and AF072247, respectively. The in-frame spliced form MBD3b only contains part of the MBD. (B) Sequences and splicing junctions of the MBD3a and MBD3b. The orange nucleotides that encode 5′ portion of MBD are spliced out in MBD3b. The peptide sequence derived from the band labeled as MBD3a in Fig. 1A is underlined. Other peptides derived from the MBD3 bands of Fig. 1A have perfect match to MBD3: KQEELVQQVR, TMDLPK, GKPDLNTALPVR, and NPGVWLNTTQPLCK. (C) MTA2 is related to the metastasis-associated protein MTA1. Amino acid sequence alignment of human MTA2 (GenBank accession no. AB016591), mouse MTA2 (GenBank accession no. AF159259) with human MTA1 (GenBank accession no. U35113). Sequence alignment was performed using Gap of the GCG program (University of Wisconsin, Madison). Peptide sequences obtained from microsequencing of the band labeled MTA2 in Fig. 1A are underlined. The putative zinc-finger is indicated by green triangles. The putative leucine zipper is indicated by purple dots. The yellow box indicates a potential tyrosine kinase phosphorylation site. Human and mouse MTA2 are 98% identical and are 65% and 63% identical to human MTA1, respectively.
