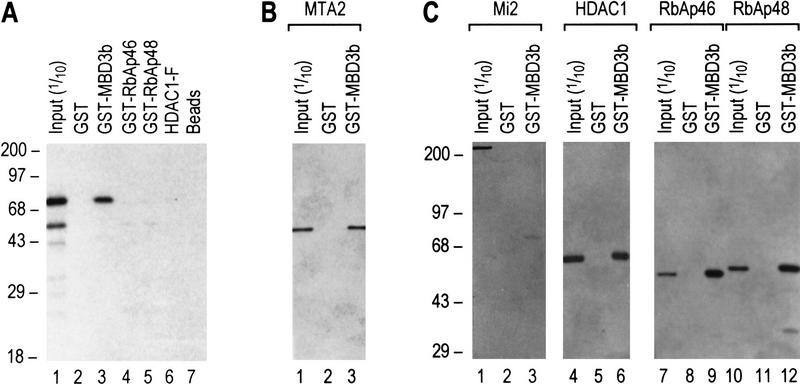
Figure 4.
MBD3 interacts with MTA2 and components of the histone deacetylase core complex. (A) GST pull-down assays show that in vitro-translated MTA2 only interacts with MBD3. Equal amounts (5 μl) of in vitro-translated and labeled MTA2 was incubated with 10 μl of glutathione–agarose beads or anti-Flag beads coated with 2 μg of proteins as indicated at the top. After extensive wash, bound proteins were eluted, resolved by SDS-PAGE, and visualized by autoradiography. Ten percent of input was loaded on lane 1. (B) MTA2 directly interacts with MBD3. An experimental procedure similar to A was used except that MTA2 was purified from Escherichia coli (Fig. 3D, lane 3). Bound proteins were eluted, resolved by SDS-PAGE, and visualized by Western blot. (C) MBD3 interacts with components of the core complex in a GST pull-down assay. Assays were performed as in B. Input proteins are indicated at top and are the same as those used in Fig. 3D.