Figure 1.
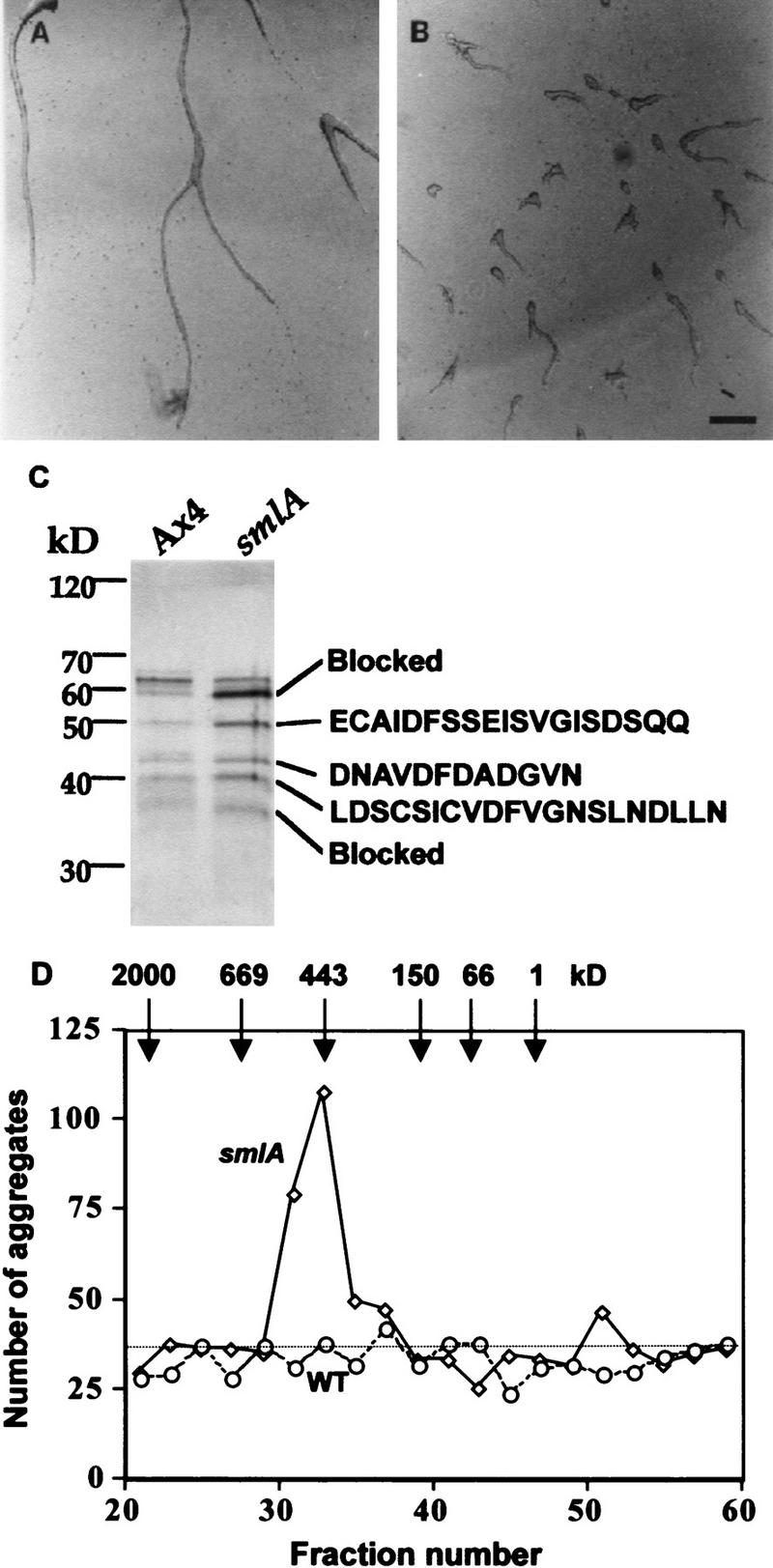
Purification of the counting factor. (A,B) Bioassay of the purified counting factor activity. Fractions 18–23 from the hydroxyapatite column were pooled, concentrated, desalted, and run on a 10% nondenaturing polyacrylamide gel. Then, 0.5-cm slices were excised, electroeluted, and concentrated. Ax4 cells were starved in submerged culture for 15 hr in the presence of (A) the material eluted from slice 3 of the nondenaturing gel of Ax4 CM, (B) material eluted from the same slice from smlA CM. Slice 2 from the smlA preparation had a similar number of small aggregates as seen for slice 3 from smlA; all other slices from the smlA prep and all the slices from the Ax4 prep had normal aggregation streams as shown for the Ax4 slice 2. Bar in B, 200 μm. (C) Silver-stained SDS–polyacrylamide gel of the combined material eluted from gel slices 2 and 3. In other experiments, the polypeptide composition of the material from the two slices was seen to be identical. The amino-terminal amino acid sequences of the proteins are shown at right. The sequence we obtained for the 43-kD band was a mixture of two sequences; the sequence of the more abundant polypeptide is shown. (D) Gel-filtration chromatography of the active fractions from the hydroxylapatite column. (Solid line) Number of aggregates formed in the presence of fractionated smlA; (broken line) wild-type (WT) control; (dotted line) number of aggregates formed in PBM.
