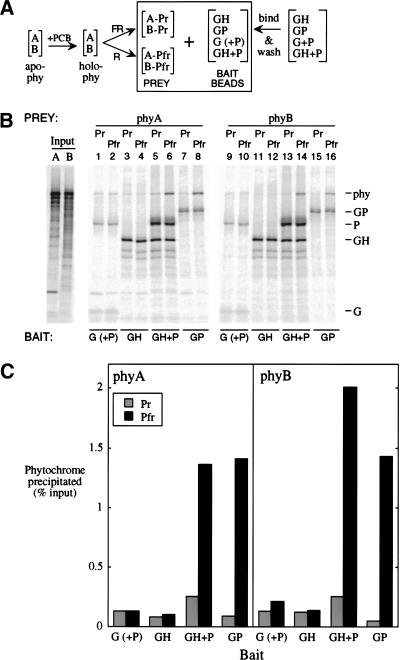
Figure 8.
Both phyA and phyB bind as Pfr in vitro to both the PIF3 homodimer and a HFR1/PIF3 complex. (A) PhyA or phyB apoproteins were expressed separately in vitro (apo-phy), and combined with chromophore (PCB) to make spectrally active holoprotein (holo-phy); portions of each phytochrome solution were then irradiated with either FR or R to form predominantly Pr (A-Pr; B-Pr) or Pfr (A-Pfr; B-Pfr) forms, respectively. Bait beads were prepared by binding GAD-tagged proteins (G; alone or coexpressed with untagged PIF3 [P]), GAD-HFR1 (GH), or GAD-PIF3 (GP) to MAb-Protein A-beads, and then washing to remove loosely bound protein. Bait proteins were labeled at 2% of the specific activity of the phytochromes. (B) Autoradiograms of proteins separated by SDS-PAGE. Input phyA (A) and phyB (B) lanes were loaded with one-fifth the fraction of each binding reaction that was loaded for washed precipitates. (C) Quantitation of the phytochrome precipitated in (B).