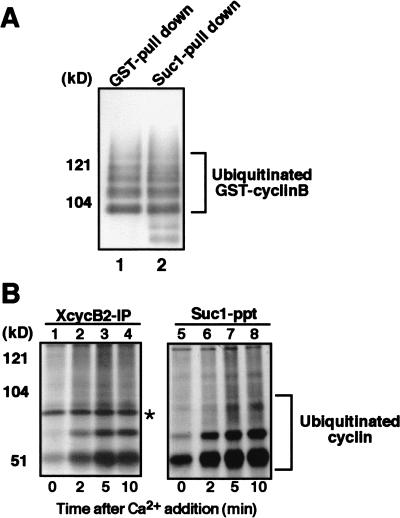
Figure 4.
Cdc2 retains its association with ubiquitinated cyclin B. (A) Cdc2 is in a complex with cyclin B that has been polyubiquitinated in vitro. Purified GST-cyclin B–Cdc2 complex was incubated with biotinylated ubiquitin, E1, E2 (hE2-C), and, as a source of APC/C, anti-Cdc27 immunoprecipitates from Xenopus egg extracts that were arrested at anaphase by the addition of calcium together with nondegradable cyclin B fragment to CSF extracts. Then, GST–cyclin B was pulled down by glutathione–Sepharose 4B, resolved by SDS-PAGE and transferred to PVDF membrane. The membrane was reacted with ExtraAvidin peroxidase at room temperature for 1 h. Ubiquitinated cyclin B was visualized by ECL (lane 1). Alternatively, Cdc2 was recovered by Suc1–Sepharose, followed by ECL detection of ubiquitination (lane 2). (B) Cdc2 is complexed with cyclin B that is ubiquitinated in Xenopus egg extracts. Biotinylated ubiquitin was added to CSF extracts and, then, cyclin B degradation was induced by the addition of calcium. Samples taken at the indicated times were subjected to immunoprecipitation for Xenopus cyclin B2 or recovery of Cdc2 by Suc1–Sepharose beads. Ubiquitinated cyclins were visualized as described in A. (Asterisk) Non-specific band.