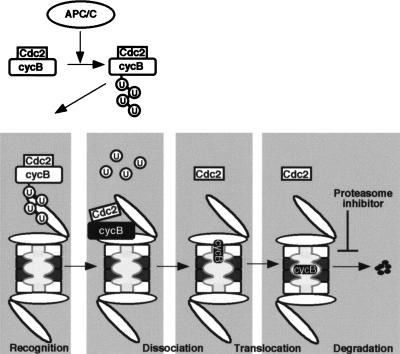
Figure 8.
A model for release of Cdc2 from cyclin B by the 26S proteasome. First, cyclin B complexed with Cdc2 is polyubiquitinated by an APC/C-dependent pathway at exit from M phase. Second, the lid of the 19S regulatory particle recognizes and tethers polyubiquitinated cyclin B that remains associated with Cdc2. Third, the cyclin B–Cdc2 complex is unfolded and dissociated by the chaperone-like activity of the base of the 19S regulatory particle with the aid of the α ring of the 20S proteasome. The translocation of unfolded cyclin B to the 20S proteasome may be required for further unfolding of cyclin B, and both processes may be coupled to each other. Finally, the unfolded cyclin B is translocated and degraded in the hollow center (β rings of the 20S proteasome) in which all catalytic sites are located. The inhibitory step by MG115 in the present study is indicated by the proteasome inhibitor.